Evaluation of the efficacy of LAMP-based SARS-CoV-2 detection with simple RNA extraction from nasopharyngeal swabs: A prospective observational study
- PMID: 34905576
- PMCID: PMC8670695
- DOI: 10.1371/journal.pone.0260732
Evaluation of the efficacy of LAMP-based SARS-CoV-2 detection with simple RNA extraction from nasopharyngeal swabs: A prospective observational study
Abstract
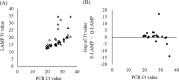
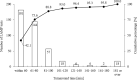
The Loopamp SARS-CoV-2 Detection Kit is used for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Loop-mediated isothermal amplification (LAMP) is based on a measurement principle that can be used with a relatively simple device. Detection using this kit requires viral RNA extraction from samples with the QIAGEN QIAamp Viral Mini Kit (QIAGEN extraction) or the Loopamp Viral RNA Extraction Kit (Eiken extraction), which are recommended by the manufacturer. However, the efficacy of LAMP-based SARS-CoV-2 detection using these extraction methods has not been compared. In this study, we aimed to compare the results of genome extraction and detection from nasopharyngeal swab samples using the QIAGEN and Eiken extraction kits. The present study involved patients who presented to the Rinku General Medical Center with suspected COVID-19 (25 positive and 26 negative cases). A comparison of the results obtained using each extraction method with those obtained via PCR showed that the positive, negative, and overall concordance rates between QIAGEN extraction and PCR were 96.0% (24/25 samples), 100% (26/26), and 98.0% (50/51; κ = 0.96, 95% CI = 0.69-1.00), respectively. Results with Eiken extraction were also favorable, with positive, negative, and overall concordance rates of 88.0% (22/25), 100% (26/26), and 94.1% (48/51; κ = 0.88, 95% CI = 0.61-1.00), respectively. Favorable results were obtained using both QIAGEN and Eiken extraction kits. Since Eiken extraction can be completed in a few minutes, it enables prompt and reliable testing for SARS-CoV-2 detection.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: assay kits used in this study were provided by Eiken Chemical Co., Ltd. under a joint research contract. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Comparison of COVID-19 laboratory diagnosis by commercial kits: Effectivity of RT-PCR to the RT-LAMP.J Med Virol. 2022 May;94(5):1998-2007. doi: 10.1002/jmv.27559. Epub 2022 Jan 21. J Med Virol. 2022. PMID: 34997587 Free PMC article.
-
Evaluation of the SARS-CoV-2 RNA detection reagent LAMPdirect Genelyzer KIT using nasopharyngeal swab and saliva samples.Diagn Microbiol Infect Dis. 2024 Jul;109(3):116297. doi: 10.1016/j.diagmicrobio.2024.116297. Epub 2024 Apr 4. Diagn Microbiol Infect Dis. 2024. PMID: 38604076
-
Clinical testing on SARS-CoV-2 swab samples using reverse-transcription loop-mediated isothermal amplification (RT-LAMP).BMC Infect Dis. 2022 Aug 18;22(1):697. doi: 10.1186/s12879-022-07684-w. BMC Infect Dis. 2022. PMID: 35982419 Free PMC article.
-
Strategies That Facilitate Extraction-Free SARS-CoV-2 Nucleic Acid Amplification Tests.Viruses. 2022 Jun 15;14(6):1311. doi: 10.3390/v14061311. Viruses. 2022. PMID: 35746782 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification (LAMP): The Better Sibling of PCR?Cells. 2021 Jul 29;10(8):1931. doi: 10.3390/cells10081931. Cells. 2021. PMID: 34440699 Free PMC article. Review.
Cited by
-
A Finger-Actuated Sample-Dosing Capillary-Driven Microfluidic Device for Loop-Mediated Isothermal Amplification.Biosensors (Basel). 2024 Aug 23;14(9):410. doi: 10.3390/bios14090410. Biosensors (Basel). 2024. PMID: 39329785 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Analytical and clinical performances of seven direct detection assays for SARS-CoV-2.J Clin Virol Plus. 2023 Feb;3(1):100138. doi: 10.1016/j.jcvp.2023.100138. Epub 2023 Jan 13. J Clin Virol Plus. 2023. PMID: 36683610 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

