Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against alpha and delta variants in patients with multiple myeloma
- PMID: 34905598
- PMCID: PMC8674107
- DOI: 10.1182/blood.2021013714
Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against alpha and delta variants in patients with multiple myeloma
Figures
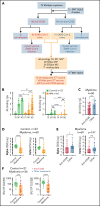

References
-
- European Society for Medical Oncology,. ESMO Statements on vaccination against COVID-19 in people with cancer. https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination. Accessed 24 June 2021.
-
- Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. - PubMed
-
- Planas D, Bruel T, Grzelak L, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27(5):917–924. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

