Hypofibrinogenemia with preserved hemostasis and protection from thrombosis in mice with an Fga truncation mutation
- PMID: 34905618
- PMCID: PMC8900273
- DOI: 10.1182/blood.2021012537
Hypofibrinogenemia with preserved hemostasis and protection from thrombosis in mice with an Fga truncation mutation
Abstract
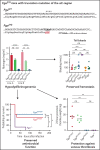
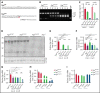
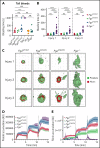
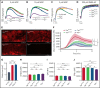
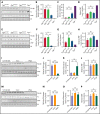
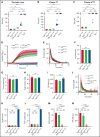
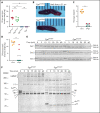
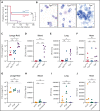
Genetic variants within the fibrinogen Aα chain encoding the αC-region commonly result in hypodysfibrinogenemia in patients. However, the (patho)physiological consequences and underlying mechanisms of such mutations remain undefined. Here, we generated Fga270 mice carrying a premature termination codon within the Fga gene at residue 271. The Fga270 mutation was compatible with Mendelian inheritance for offspring of heterozygous crosses. Adult Fga270/270 mice were hypofibrinogenemic with ∼10% plasma fibrinogen levels relative to FgaWT/WT mice, linked to 90% reduction in hepatic Fga messenger RNA (mRNA) because of nonsense-mediated decay of the mutant mRNA. Fga270/270 mice had preserved hemostatic potential in vitro and in vivo in models of tail bleeding and laser-induced saphenous vein injury, whereas Fga-/- mice had continuous bleeding. Platelets from FgaWT/WT and Fga270/270 mice displayed comparable initial aggregation following adenosine 5'-diphosphate stimulation, but Fga270/270 platelets quickly disaggregated. Despite ∼10% plasma fibrinogen, the fibrinogen level in Fga270/270 platelets was ∼30% of FgaWT/WT platelets with a compensatory increase in fibronectin. Notably, Fga270/270 mice showed complete protection from thrombosis in the inferior vena cava stasis model. In a model of Staphylococcus aureus peritonitis, Fga270/270 mice supported local, fibrinogen-mediated bacterial clearance and host survival comparable to FgaWT/WT, unlike Fga-/- mice. Decreasing the normal fibrinogen levels to ∼10% with small interfering RNA in mice also provided significant protection from venous thrombosis without compromising hemostatic potential and antimicrobial function. These findings both reveal novel molecular mechanisms underpinning fibrinogen αC-region truncation mutations and highlight the concept that selective fibrinogen reduction may be efficacious for limiting thrombosis while preserving hemostatic and immune protective functions.
© 2022 by The American Society of Hematology.
Figures

Comment in
-
Fibrinogen levels and thrombosis prevention.Blood. 2022 Mar 3;139(9):1269-1271. doi: 10.1182/blood.2021015051. Blood. 2022. PMID: 35238893 No abstract available.
References
-
- Ridgway HJ, Brennan SO, Faed JM, George PM. Fibrinogen Otago: a major alpha chain truncation associated with severe hypofibrinogenaemia and recurrent miscarriage. Br J Haematol. 1997;98(3): 632-639. - PubMed
-
- Koopman J, Haverkate F, Grimbergen J, Egbring R, Lord ST. Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acids A alpha 461-610 (Lys 461 AAA-->stop TAA). Blood. 1992;80(8):1972-1979. - PubMed
-
- Tsurupa G, Medved L. Identification and characterization of novel tPA- and plasminogen-binding sites within fibrin(ogen) alpha C-domains. Biochemistry. 2001;40(3):801-808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

