Mechanisms of Sinoatrial Node Dysfunction in Heart Failure With Preserved Ejection Fraction
- PMID: 34905696
- PMCID: PMC9083886
- DOI: 10.1161/CIRCULATIONAHA.121.054976
Mechanisms of Sinoatrial Node Dysfunction in Heart Failure With Preserved Ejection Fraction
Abstract
Background: The ability to increase heart rate during exercise and other stressors is a key homeostatic feature of the sinoatrial node (SAN). When the physiological heart rate response is blunted, chronotropic incompetence limits exercise capacity, a common problem in patients with heart failure with preserved ejection fraction (HFpEF). Despite its clinical relevance, the mechanisms of chronotropic incompetence remain unknown.
Methods: Dahl salt-sensitive rats fed a high-salt diet and C57Bl6 mice fed a high-fat diet and an inhibitor of constitutive nitric oxide synthase (Nω-nitro-L-arginine methyl ester [L-NAME]; 2-hit) were used as models of HFpEF. Myocardial infarction was created to induce HF with reduced ejection fraction. Rats and mice fed with a normal diet or those that had a sham surgery served as respective controls. A comprehensive characterization of SAN function and chronotropic response was conducted by in vivo, ex vivo, and single-cell electrophysiologic studies. RNA sequencing of SAN was performed to identify transcriptomic changes. Computational modeling of biophysically-detailed human HFpEF SAN was created.
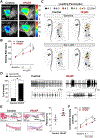
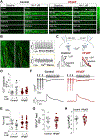
Results: Rats with phenotypically-verified HFpEF exhibited limited chronotropic response associated with intrinsic SAN dysfunction, including impaired β-adrenergic responsiveness and an alternating leading pacemaker within the SAN. Prolonged SAN recovery time and reduced SAN sensitivity to isoproterenol were confirmed in the 2-hit mouse model. Adenosine challenge unmasked conduction blocks within the SAN, which were associated with structural remodeling. Chronotropic incompetence and SAN dysfunction were also found in rats with HF with reduced ejection fraction. Single-cell studies and transcriptomic profiling revealed HFpEF-related alterations in both the "membrane clock" (ion channels) and the "Ca2+ clock" (spontaneous Ca2+ release events). The physiologic impairments were reproduced in silico by empirically-constrained quantitative modeling of human SAN function.
Conclusions: Chronotropic incompetence and SAN dysfunction were seen in both models of HF. We identified that intrinsic abnormalities of SAN structure and function underlie the chronotropic response in HFpEF.
Keywords: arrhythmias, cardiac; heart failure; heart rate; sinoatrial node.
Figures




References
-
- Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

