Comparison of plasma neurofilament light and total tau as neurodegeneration markers: associations with cognitive and neuroimaging outcomes
- PMID: 34906229
- PMCID: PMC8672619
- DOI: 10.1186/s13195-021-00944-y
Comparison of plasma neurofilament light and total tau as neurodegeneration markers: associations with cognitive and neuroimaging outcomes
Abstract
Background: Total tau protein (T-Tau) and neurofilament light chain (NfL) have emerged as candidate plasma biomarkers of neurodegeneration, but studies have not compared how these biomarkers cross-sectionally or longitudinally associate with cognitive and neuroimaging measures. We therefore compared plasma T-Tau and NfL as cross-sectional and longitudinal markers of (1) global and domain-specific cognitive decline and (2) neuroimaging markers of cortical thickness, hippocampal volume, white matter integrity, and white matter hyperintensity volume.
Methods: We included 995 participants without dementia who were enrolled in the Mayo Clinic Study of Aging cohort. All had concurrent plasma NfL and T-tau, cognitive status, and neuroimaging data. Follow-up was repeated approximately every 15 months for a median of 6.2 years. Plasma NfL and T-tau were measured on the Simoa-HD1 Platform. Linear mixed effects models adjusted for age, sex, and education examined associations between baseline z-scored plasma NfL or T-tau and cognitive or neuroimaging outcomes. Analyses were replicated in Alzheimer's Disease Neuroimaging Initiative (ADNI) among 387 participants without dementia followed for a median of 3.0 years.
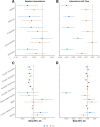
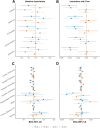
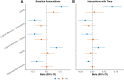
Results: At baseline, plasma NfL was more strongly associated with all cognitive and neuroimaging outcomes. The combination of having both elevated NfL and T-tau at baseline, compared to elevated levels of either alone, was more strongly associated at cross-section with worse global cognition and memory, and with neuroimaging measures including temporal cortex thickness and increased number of infarcts. In longitudinal analyses, baseline plasma T-tau did not add to the prognostic value of baseline plasma NfL. Results using ADNI data were similar.
Conclusions: Our results indicate plasma NfL had better utility as a prognostic marker of cognitive decline and neuroimaging changes. Plasma T-tau added cross-sectional value to NfL in specific contexts.
Trial registration: Not applicable.
Keywords: Blood-based biomarker; Cognition; Neurofilament light chain; Neuroimaging; Total tau.
© 2021. The Author(s).
Conflict of interest statement
Dr. Knopman served on a Data Safety Monitoring Board for the DIAN study. He serves on a Data Safety monitoring Board for a tau therapeutic for Biogen, but receives no personal compensation. He is a site investigator in the Biogen aducanumab trials. He is an investigator in a clinical trial sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche and Alzeca Biosciences but receives no personal compensation. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Philips Molecular lmaging, Life Molecular lmaging, AVID Radiopharamceuticals, and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, and AVID Radiopharmaceuticals. Dr. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. Dr. Petersen is a consultant for Roche, Biogen, Merck, Eli Lilly, and Genentech. He receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003). Dr. Mielke is a consultant for Biogen, Brain Protection Company, and LabCorp on blood-based biomarkers. She serves on Editorial Boards for
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

