COVID-19 Vaccine-Associated Subclinical Axillary Lymphadenopathy on Screening Mammogram
- PMID: 34906409
- PMCID: PMC8595349
- DOI: 10.1016/j.acra.2021.11.010
COVID-19 Vaccine-Associated Subclinical Axillary Lymphadenopathy on Screening Mammogram
Erratum in
-
Erratum to 'COVID-19 vaccine-associated subclinical axillary lymphadenopathy on screening mammogram' [Academic Radiology Volume 29, Issue 4, April 2022, Pages 501-507].Acad Radiol. 2022 Jun;29(6):950. doi: 10.1016/j.acra.2022.03.029. Epub 2022 Apr 8. Acad Radiol. 2022. PMID: 35410800 Free PMC article. No abstract available.
Abstract
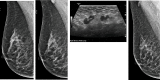
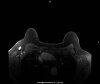
Background: Women who received a COVID-19 vaccination may display subclinical unilateral axillary lymphadenopathy on screening mammography, which can appear suspicious for malignancy, leading to additional diagnostic evaluation.
Purpose: To evaluate the prevalence of subclinical unilateral axillary lymphadenopathy (sLAD) on screening mammogram in women who received either the first or second dose of the Pfizer-BioNTech (Pfizer) or Moderna COVID-19 vaccines compared to women who have not.
Materials and methods: In this IRB-approved, HIPAA complaint study from 12/14/2020 to 4/14/2021, 1027 patients presented for screening mammography and met study inclusion criteria. Patients with history of baseline lymphadenopathy or prior cancer diagnosis were excluded.
Results: Of the 1027 women, 43 were recalled for unilateral sLAD. 34 women received a COVID-19 vaccination ipsilateral to the sLAD (Pfizer n=19, 44.2%; Moderna n=15, 34.9%), 9 did not (20.9%). Incidence of unilateral axillary sLAD was significantly higher (p-value<0.01) in those who received a COVID-19 vaccination within approximately 7 weeks preceding screening mammogram. 13.2% of patients who received the Pfizer vaccine and 9.5% of patients who received the Moderna vaccine developed sLAD. Moderna's vaccine elicited a more robust reaction in the elderly (Moderna 63.7 years vs. Pfizer 59.7 years). For both vaccines, sLAD resolved on average 46.5 days after the last COVID-19 vaccine (p=0.44).
Conclusion: Women who have received either mRNA COVID-19 vaccines may benefit from scheduling their screening mammogram before vaccination or consider delaying screening mammography 8 weeks. While Pfizer may have an overall more robust immune response, Moderna may elicit a stronger immune response in elderly women.
Summary: Women who received a COVID-19 vaccination before screening mammography were significantly more likely to present with subclinical axillary lymphadenopathy than women who did not receive the vaccine.
Key results: 13.2% of women who received a Pfizer-BioNTech vaccine exhibited subclinical axillary lymphadenopathy compared to 9.5% of those who received the Moderna vaccine. Only 1.2 % of those who did not receive a vaccine presented with subclinical unilateral axillary lymphadenopathy. The average time of resolution of the lymphadenopathy on diagnostic mammogram was 46.5 days overall, with Pfizer-BioNTech taking 50.7 days and Moderna 41.5 days.
Copyright © 2021. Published by Elsevier Inc.
Figures
Comment in
-
COVID-19 Vaccination and Subclinical Axillary Lymphadenopathy on Mammogram: Correspondence.Acad Radiol. 2022 Apr;29(4):633. doi: 10.1016/j.acra.2021.12.024. Epub 2021 Dec 27. Acad Radiol. 2022. PMID: 35058134 Free PMC article. No abstract available.
-
Imaging of axillary lymph nodes in the COVID-19 era: A lesson to be learned.Eur J Radiol. 2022 Sep;154:110446. doi: 10.1016/j.ejrad.2022.110446. Epub 2022 Jul 21. Eur J Radiol. 2022. PMID: 35917755 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

