Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques
- PMID: 34906975
- PMCID: PMC8865895
- DOI: 10.1212/WNL.0000000000013211
Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques
Abstract
Background and objectives: To determine the diagnostic accuracy of a plasma Aβ42/Aβ40 assay in classifying amyloid PET status across global research studies using samples collected by multiple centers that utilize different blood collection and processing protocols.
Methods: Plasma samples (n = 465) were obtained from 3 large Alzheimer disease (AD) research cohorts in the United States (n = 182), Australia (n = 183), and Sweden (n = 100). Plasma Aβ42/Aβ40 was measured by a high precision immunoprecipitation mass spectrometry (IPMS) assay and compared to the reference standards of amyloid PET and CSF Aβ42/Aβ40.
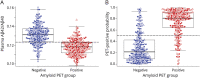
Results: In the combined cohort of 465 participants, plasma Aβ42/Aβ40 had good concordance with amyloid PET status (receiver operating characteristic area under the curve [AUC] 0.84, 95% confidence interval [CI] 0.80-0.87); concordance improved with the inclusion of APOE ε4 carrier status (AUC 0.88, 95% CI 0.85-0.91). The AUC of plasma Aβ42/Aβ40 with CSF amyloid status was 0.85 (95% CI 0.78-0.91) and improved to 0.93 (95% CI 0.89-0.97) with APOE ε4 status. These findings were consistent across the 3 cohorts, despite differences in protocols. The assay performed similarly in both cognitively unimpaired and impaired individuals.
Discussion: Plasma Aβ42/Aβ40 is a robust measure for detecting amyloid plaques and can be utilized to aid in the diagnosis of AD, identify those at risk for future dementia due to AD, and improve the diversity of populations enrolled in AD research and clinical trials.
Classification of evidence: This study provides Class II evidence that plasma Aβ42/Aβ40, as measured by a high precision IPMS assay, accurately diagnoses brain amyloidosis in both cognitively unimpaired and impaired research participants.
© 2021 American Academy of Neurology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
