Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD: A Randomized Controlled Trial
- PMID: 34907021
- PMCID: PMC8823928
- DOI: 10.2215/CJN.08950621
Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD: A Randomized Controlled Trial
Erratum in
-
Correction: Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD: A Randomized Controlled Trial.Clin J Am Soc Nephrol. 2022 Jun;17(6):877-878. doi: 10.2215/CJN.03710322. Epub 2022 May 20. Clin J Am Soc Nephrol. 2022. PMID: 35595530 Free PMC article. No abstract available.
Abstract
Background and objectives: Clinical manifestations of autosomal dominant polycystic kidney disease (ADPKD), including evidence of vascular dysfunction, can begin in childhood. Curcumin is a polyphenol found in turmeric that reduces vascular dysfunction in rodent models and humans without ADPKD. It also slows kidney cystic progression in a murine model of ADPKD. We hypothesized that oral curcumin therapy would reduce vascular endothelial dysfunction and arterial stiffness in children/young adults with ADPKD.
Design, setting, participants, & measurements: In a randomized, placebo-controlled, double-blind trial, 68 children/young adults 6-25 years of age with ADPKD and eGFR>80 ml/min per 1.73 m2 were randomized to either curcumin supplementation (25 mg/kg body weight per day) or placebo administered in powder form for 12 months. The coprimary outcomes were brachial artery flow-mediated dilation and aortic pulse-wave velocity. We also assessed change in circulating/urine biomarkers of oxidative stress/inflammation and kidney growth (height-adjusted total kidney volume) by magnetic resonance imaging. In a subgroup of participants ≥18 years, vascular oxidative stress was measured as the change in brachial artery flow-mediated dilation following an acute infusion of ascorbic acid.
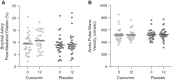
Results: Enrolled participants were 18±5 (mean ± SD) years, 54% were girls, baseline brachial artery flow-mediated dilation was 9.3±4.1% change, and baseline aortic pulse-wave velocity was 512±94 cm/s. Fifty-seven participants completed the trial. Neither coprimary end point changed with curcumin (estimated change [95% confidence interval] for brachial artery flow-mediated dilation [percentage change]: curcumin: 1.14; 95% confidence interval, -0.84 to 3.13; placebo: 0.33; 95% confidence interval, -1.34 to 2.00; estimated difference for change: 0.81; 95% confidence interval, -1.21 to 2.84; P=0.48; aortic pulse-wave velocity [centimeters per second]: curcumin: 0.6; 95% confidence interval, -25.7 to 26.9; placebo: 6.5; 95% confidence interval, -20.4 to 33.5; estimated difference for change: -5.9; 95% confidence interval, -35.8 to 24.0; P=0.67; intent to treat). There was no curcumin-specific reduction in vascular oxidative stress or changes in mechanistic biomarkers. Height-adjusted total kidney volume also did not change as compared with placebo.
Conclusions: Curcumin supplementation does not improve vascular function or slow kidney growth in children/young adults with ADPKD.
Clinical trial registry name and registration number: Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD, NCT02494141.
Podcast: This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_02_07_CJN08950621.mp3.
Keywords: ADPKD; aging; endothelial cells; oxidative stress; pulse wave velocity.
Copyright © 2022 by the American Society of Nephrology.
Figures




References
-
- Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 - PubMed
-
- Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 - PubMed
-
- Ivy DD, Shaffer EM, Johnson AM, Kimberling WJ, Dobin A, Gabow PA: Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2032–2036, 1995 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

