A low-cost and shielding-free ultra-low-field brain MRI scanner
- PMID: 34907181
- PMCID: PMC8671508
- DOI: 10.1038/s41467-021-27317-1
A low-cost and shielding-free ultra-low-field brain MRI scanner
Abstract
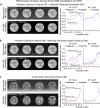
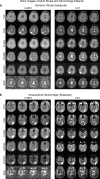
Magnetic resonance imaging is a key diagnostic tool in modern healthcare, yet it can be cost-prohibitive given the high installation, maintenance and operation costs of the machinery. There are approximately seven scanners per million inhabitants and over 90% are concentrated in high-income countries. We describe an ultra-low-field brain MRI scanner that operates using a standard AC power outlet and is low cost to build. Using a permanent 0.055 Tesla Samarium-cobalt magnet and deep learning for cancellation of electromagnetic interference, it requires neither magnetic nor radiofrequency shielding cages. The scanner is compact, mobile, and acoustically quiet during scanning. We implement four standard clinical neuroimaging protocols (T1- and T2-weighted, fluid-attenuated inversion recovery like, and diffusion-weighted imaging) on this system, and demonstrate preliminary feasibility in diagnosing brain tumor and stroke. Such technology has the potential to meet clinical needs at point of care or in low and middle income countries.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Fuchs VR, Sox HC., Jr. Physicians’ views of the relative importance of thirty medical innovations. Health Aff. 2001;20:30–42. - PubMed
-
- Magnetic resonance imaging (MRI) exams (indicator). Vol. 2021 (OECD).
-
- Reimer, P. Clinical MR imaging: a practical approach (Springer, Berlin, 2010).
-
- Nowogrodzki A. The world’s strongest MRI machines are pushing human imaging to new limits. Nature. 2018;563:24–26. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

