Calibration of two validated SARS-CoV-2 pseudovirus neutralization assays for COVID-19 vaccine evaluation
- PMID: 34907214
- PMCID: PMC8671391
- DOI: 10.1038/s41598-021-03154-6
Calibration of two validated SARS-CoV-2 pseudovirus neutralization assays for COVID-19 vaccine evaluation
Abstract
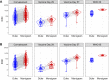
Vaccine-induced neutralizing antibodies (nAbs) are key biomarkers considered to be associated with vaccine efficacy. In United States government-sponsored phase 3 efficacy trials of COVID-19 vaccines, nAbs are measured by two different validated pseudovirus-based SARS-CoV-2 neutralization assays, with each trial using one of the two assays. Here we describe and compare the nAb titers obtained in the two assays. We observe that one assay consistently yielded higher nAb titers than the other when both assays were performed on the World Health Organization's anti-SARS-CoV-2 immunoglobulin International Standard, COVID-19 convalescent sera, and mRNA-1273 vaccinee sera. To overcome the challenge this difference in readout poses in comparing/combining data from the two assays, we evaluate three calibration approaches and show that readouts from the two assays can be calibrated to a common scale. These results may aid decision-making based on data from these assays for the evaluation and licensure of new or adapted COVID-19 vaccines.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Calibration of Two Validated SARS-CoV-2 Pseudovirus Neutralization Assays for COVID-19 Vaccine Evaluation.Res Sq [Preprint]. 2021 Sep 2:rs.3.rs-862572. doi: 10.21203/rs.3.rs-862572/v1. Res Sq. 2021. Update in: Sci Rep. 2021 Dec 14;11(1):23921. doi: 10.1038/s41598-021-03154-6. PMID: 34494017 Free PMC article. Updated. Preprint.
-
Calibration of Two Validated SARS-CoV-2 Pseudovirus Neutralization Assays for COVID-19 Vaccine Evaluation.medRxiv [Preprint]. 2021 Sep 14:2021.09.09.21263049. doi: 10.1101/2021.09.09.21263049. medRxiv. 2021. Update in: Sci Rep. 2021 Dec 14;11(1):23921. doi: 10.1038/s41598-021-03154-6. PMID: 34545372 Free PMC article. Updated. Preprint.
References
-
- McGill COVID19 Vaccine Tracker Team COVID-19 Vaccine Tracker. https://covid19.trackvaccines.org/ Last updated 5 Mar, 2021. Access date 8 Mar, 2021.
Publication types
MeSH terms
Substances
Grants and funding
- UM1AI068614/National Institute of Allergy and Infectious Diseases
- S10 AI174104/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- 75N93019C00050/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1AI068618/National Institute of Allergy and Infectious Diseases
- U01 AI149644/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1AI068635/National Institute of Allergy and Infectious Diseases
- HHSN272201500002C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

