Current status and challenges in the drug treatment for fibrotic nonalcoholic steatohepatitis
- PMID: 34907360
- PMCID: PMC9061812
- DOI: 10.1038/s41401-021-00822-1
Current status and challenges in the drug treatment for fibrotic nonalcoholic steatohepatitis
Abstract
Currently, nonalcoholic steatohepatitis (NASH) is one of the most common forms of chronic hepatitis, increasing the burden of health care worldwide. In patients with NASH, the fibrosis stage is the most predictive factor of long-term events. However, there are still no drugs approved by the Food and Drug Administration of the United States for treating biopsy-proven NASH with fibrosis or cirrhosis. Although some novel drugs have shown promise in preclinical studies and led to improvement in terms of hepatic fat content and steatohepatitis, a considerable proportion of them have failed to achieve histological endpoints of fibrosis improvement. Due to the large number of NASH patients and adverse clinical outcomes, the search for novel drugs is necessary. In this review, we discuss current definitions for the evaluation of treatment efficacy in fibrosis improvement for NASH patients, and we summarize novel agents in the pipeline from different mechanisms and phases of trial. We also critically review the challenges we face in the development of novel agents for fibrotic NASH and NASH cirrhosis.
Keywords: cirrhosis; liver fibrosis; nonalcoholic steatohepatitis; novel therapies; treatment efficacy.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures

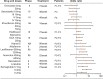
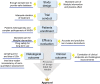
Similar articles
-
The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis.Contemp Clin Trials. 2021 May;104:106335. doi: 10.1016/j.cct.2021.106335. Epub 2021 Feb 28. Contemp Clin Trials. 2021. PMID: 33657443 Clinical Trial.
-
Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials.Hepatology. 2019 Aug;70(2):522-531. doi: 10.1002/hep.30418. Epub 2019 Mar 7. Hepatology. 2019. PMID: 30549292 Free PMC article.
-
Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis.Gastroenterology. 2021 Jan;160(1):219-231.e1. doi: 10.1053/j.gastro.2020.08.004. Epub 2020 Aug 8. Gastroenterology. 2021. PMID: 32781086 Clinical Trial.
-
Nonalcoholic Steatohepatitis (NASH) and Hepatic Fibrosis: Emerging Therapies.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:649-662. doi: 10.1146/annurev-pharmtox-010617-052545. Epub 2017 Oct 20. Annu Rev Pharmacol Toxicol. 2018. PMID: 29058997 Review.
-
Emerging drugs for the treatment of hepatic fibrosis on nonalcoholic steatohepatitis.Expert Opin Emerg Drugs. 2024 Jun;29(2):127-137. doi: 10.1080/14728214.2024.2328036. Epub 2024 Mar 25. Expert Opin Emerg Drugs. 2024. PMID: 38469871 Review.
Cited by
-
The genetic background shapes the susceptibility to mitochondrial dysfunction and NASH progression.J Exp Med. 2023 Apr 3;220(4):e20221738. doi: 10.1084/jem.20221738. Epub 2023 Feb 14. J Exp Med. 2023. PMID: 36787127 Free PMC article.
-
Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH).Signal Transduct Target Ther. 2022 Aug 13;7(1):287. doi: 10.1038/s41392-022-01119-3. Signal Transduct Target Ther. 2022. PMID: 35963848 Free PMC article. Review.
-
All about NASH: disease biology, targets, and opportunities on the road to NASH drugs.Acta Pharmacol Sin. 2022 May;43(5):1101-1102. doi: 10.1038/s41401-022-00900-y. Epub 2022 Apr 4. Acta Pharmacol Sin. 2022. PMID: 35379932 Free PMC article. No abstract available.
-
Pathogenesis and research progress of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Hepatol. 2024 Oct 27;16(10):1142-1150. doi: 10.4254/wjh.v16.i10.1142. World J Hepatol. 2024. PMID: 39474575 Free PMC article.
References
-
- European Association for the Study of the Liver (EASL)., European Association for the Study of Diabetes (EASD)., European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

