Development and validation of an LC-MS/MS method for the quantitation of 30 legacy and emerging per- and polyfluoroalkyl substances (PFASs) in human plasma, including HFPO-DA, DONA, and cC6O4
- PMID: 34907451
- PMCID: PMC8760233
- DOI: 10.1007/s00216-021-03762-1
Development and validation of an LC-MS/MS method for the quantitation of 30 legacy and emerging per- and polyfluoroalkyl substances (PFASs) in human plasma, including HFPO-DA, DONA, and cC6O4
Erratum in
-
Correction to: Development and validation of an LC-MS/MS method for the quantitation of 30 legacy and emerging per- and polyfluoroalkyl substances (PFASs) in human plasma, including HFPO‑DA, DONA, and cC6O4.Anal Bioanal Chem. 2022 Mar;414(6):2315. doi: 10.1007/s00216-022-03873-3. Anal Bioanal Chem. 2022. PMID: 35031817 Free PMC article. No abstract available.
Abstract
Per- and polyfluoroalkyl substances (PFASs) include persistent organic pollutants whose spread is still ubiquitous. Efforts to substitute substances of high concern with fluorinated alternatives, such as HFPO-DA (GenX), DONA (ADONA), and cC6O4, have been made. The aim of this work was to develop and validate an isotopic dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) method suitable to quantify 30 PFASs in human plasma. Analytes included legacy PFASs (PFOA, PFOS, and PFHxS), fluorinated alternatives (PFBA, PFBS, 6:2 FTSA, HFPO-DA, DONA, and cC6O4), and newly identified compounds (F-53B and PFECHS). The sample preparation was rapid and consisted of simple protein precipitation and centrifugation. Calibration standards and quality control solutions were prepared with a human pooled plasma containing relatively low background levels of the considered analytes. A complete validation was carried out: the lower limits of quantitation (LLOQs) ranged from 0.009 to 0.245 µg/L; suitable linearity (determination coefficients, R2 0.989-0.999), precision (2.0-19.5%, relative standard deviation), and accuracy (87.9-113.1% of theoretical) were obtained for considered concentration ranges. No significant variations of analyte responses were recorded under investigated storage conditions and during matrix effect tests. The external verification confirmed the accuracy of the method, although limited to 12 analytes. The method was also applied to 38 human plasma samples to confirm its applicability. The developed assay is suitable for large-scale analyses of a wide range of legacy and emerging PFASs in human plasma. To our knowledge, this is the first published method including cC6O4 for human biomonitoring.
Keywords: Emerging PFAS; Fluorinated alternatives; LC-MS/MS; PFAS; Per-/polyfluoroalkyl substances; Per/polyfluoroalkyl acids.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
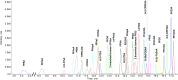

References
-
- Stockholm Convention (2020) All POPs listed in the Stockholm Convention. http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Defau.... Accessed 7 Jul 2021
-
- ECHA. Agreement of the member state committee on the identification of perfluorohexane-1-sulphonic acid and its salts as substances of very high concern. https://echa.europa.eu/documents/10162/e31ae243-0978-dd9f-23ef-38d9a743757f. 2017. Accessed 21 Oct 2020.
-
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. - DOI - PMC - PubMed
-
- ECHA. Agreement of the member state committee on the identification of perfluorononan-1-oic-acid and its sodium and ammonium salts as a substance of very high concern. 2015. https://echa.europa.eu/documents/10162/5cd2aecb-21e6-526e-b0c8-96d8ab45d5aa. Accessed 21 Oct 2020.

