MAB21L4 regulates the TGF-β-induced expression of target genes in epidermal keratinocytes
- PMID: 34908107
- PMCID: PMC8969751
- DOI: 10.1093/jb/mvab141
MAB21L4 regulates the TGF-β-induced expression of target genes in epidermal keratinocytes
Erratum in
-
Erratum.J Biochem. 2022 Mar 31;171(4):469. doi: 10.1093/jb/mvac016. J Biochem. 2022. PMID: 35181785 Free PMC article. No abstract available.
Abstract
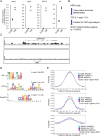
Smad proteins transduce signals downstream of transforming growth factor-β (TGF-β) and are one of the factors that regulate the expression of genes related to diseases affecting the skin. In the present study, we identified MAB21L4, also known as male abnormal 21 like 4 or C2orf54, as the most up-regulated targets of TGF-β and Smad3 in differentiated human progenitor epidermal keratinocytes using chromatin immunoprecipitation sequencing (ChIP-seq) and RNA sequencing (RNA-seq). We found that TGF-β induced expression of the barrier protein involucrin (encoded by the IVL gene). Transcriptional activity of the IVL promoter induced by TGF-β was inhibited by MAB21L4 siRNAs. Further analysis revealed that MAB21L4 siRNAs also down-regulated the expression of several target genes of TGF-β. MAB21L4 protein was located mainly in the cytosol, where it was physically bound to Smad3 and a transcriptional corepressor c-Ski. siRNAs for MAB21L4 did not inhibit the binding of Smad3 to their target genomic regions but down-regulated the acetylation of histone H3 lys 27 (H3K27ac), an active histone mark, near the Smad3 binding regions. These findings suggest that TGF-β-induced MAB21L4 up-regulates the gene expression induced by TGF-β, possibly through the inhibition of c-Ski via physical interaction in the cytosol.
Keywords: ChIP-seq; Smad3; TGF-β; c-Ski; epidermal keratinocytes.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Japanese Biochemical Society.
Figures






References
-
- Candi, E., Schmidt, R., and Melino, G. (2005) The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 - PubMed
-
- Nagano, Y., Mavrakis, K.J., Lee, K.L., Fujii, T., Koinuma, D., Sase, H., Yuki, K., Isogaya, K., Saitoh, M., Imamura, T., Episkopou, V., Miyazono, K., and Miyazawa, K. (2007) Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-beta signaling. J. Biol. Chem. 282, 20492–20501 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

