In situ spatial glycomic imaging of mouse and human Alzheimer's disease brains
- PMID: 34908231
- PMCID: PMC9198106
- DOI: 10.1002/alz.12523
In situ spatial glycomic imaging of mouse and human Alzheimer's disease brains
Abstract
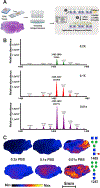
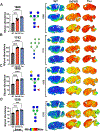
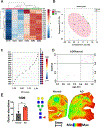
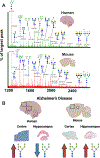
N-linked protein glycosylation in the brain is an understudied facet of glucose utilization that impacts a myriad of cellular processes including resting membrane potential, axon firing, and synaptic vesicle trafficking. Currently, a spatial map of N-linked glycans within the normal and Alzheimer's disease (AD) human brain does not exist. A comprehensive analysis of the spatial N-linked glycome would improve our understanding of brain energy metabolism, linking metabolism to signaling events perturbed during AD progression, and could illuminate new therapeutic strategies. Herein we report an optimized in situ workflow for enzyme-assisted, matrix-assisted laser desorption and ionization (MALDI) mass spectrometry imaging (MSI) of brain N-linked glycans. Using this workflow, we spatially interrogated N-linked glycan heterogeneity in both mouse and human AD brains and their respective age-matched controls. We identified robust regional-specific N-linked glycan changes associated with AD in mice and humans. These data suggest that N-linked glycan dysregulation could be an underpinning of AD pathologies.
Keywords: MALDI imaging; N-linked glycosylation; bioenergetics; carbohydrate metabolism; neuronal function; synaptic transmission.
© 2021 the Alzheimer's Association.
Conflict of interest statement
CONFLICTS OF INTEREST
Ramon C. Sun has research support and has received a consultancy fee from Maze Therapeutics. Derek B. Allison received a book royalty from Wolters Kluwer. Matthew S. Gentry has received research support and research compounds from Maze Therapeutics, Valerion Therapeutics, and Ionis Pharmaceuticals. Matthew S. Gentry also received a consultancy fee from Maze Therapeutics, PTC Therapeutics, and the Glut1-Deficiency Syndrome Foundation. Tara R. Hawkinson, Lyndsay E. A. Yong, Kia H. Markussen, Lindsey R. Conroy, Kayla M. Kerch, Lance A. Johnson, Peter T. Nelson, Chi Wang, and Harrison A. Clarke report no disclosures.
Figures


References
-
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. - PubMed
-
- Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. - PubMed
-
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

