Discovery of Novel Dual-Target Inhibitor of Bromodomain-Containing Protein 4/Casein Kinase 2 Inducing Apoptosis and Autophagy-Associated Cell Death for Triple-Negative Breast Cancer Therapy
- PMID: 34908415
- PMCID: PMC10118286
- DOI: 10.1021/acs.jmedchem.1c01382
Discovery of Novel Dual-Target Inhibitor of Bromodomain-Containing Protein 4/Casein Kinase 2 Inducing Apoptosis and Autophagy-Associated Cell Death for Triple-Negative Breast Cancer Therapy
Abstract
Bromodomain-containing protein 4 (BRD4) is an attractive epigenetic target in human cancers. Inhibiting the phosphorylation of BRD4 by casein kinase 2 (CK2) is a potential strategy to overcome drug resistance in cancer therapy. The present study describes the synthesis of multiple BRD4-CK2 dual inhibitors based on rational drug design, structure-activity relationship, and in vitro and in vivo evaluations, and 44e was identified to possess potent and balanced activities against BRD4 (IC50 = 180 nM) and CK2 (IC50 = 230 nM). In vitro experiments show that 44e could inhibit the proliferation and induce apoptosis and autophagy-associated cell death of MDA-MB-231 and MDA-MB-468 cells. In two in vivo xenograft mouse models, 44e displays potent anticancer activity without obvious toxicities. Taken together, we successfully synthesized the first highly effective BRD4-CK2 dual inhibitor, which is expected to be an attractive therapeutic strategy for triple-negative breast cancer (TNBC).
Figures
















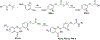
References
-
- Siegel RL; Miller KD; Jemal A Cancer statistics, 2020. CA-Cancer. Ca-Cancer J. Clin 2020, 70, 7–30. - PubMed
-
- Xu T; Zhang JF; Yang CC; Pluta R; Wang G; Ye TH; Ouyang L Identifification and optimization of 3-bromo-N’-(4-hydroxybenzylidene)-4-methylbenzohydrazide derivatives as mTOR inhibitors that induce autophagic cell death and apoptosis in triplenegative breast cancer. Eur. J. Med. Chem 2021, 219, 113424. - PubMed
-
- Franzoi MA; Romano E; Piccart M Immunotherapy for early breast cancer: too soon, too superficial, or just right? Ann. Oncol 2021, 32, 323–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

