Why stem/progenitor cells lose their regenerative potential
- PMID: 34909119
- PMCID: PMC8641024
- DOI: 10.4252/wjsc.v13.i11.1714
Why stem/progenitor cells lose their regenerative potential
Abstract
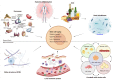
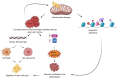
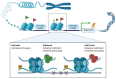
Nowadays, it is clear that adult stem cells, also called as tissue stem cells, play a central role to repair and maintain the tissue in which they reside by their self-renewal ability and capacity of differentiating into distinct and specialized cells. As stem cells age, their renewal ability declines and their capacity to maintain organ homeostasis and regeneration is impaired. From a molecular perspective, these changes in stem cells properties can be due to several types of cell intrinsic injury and DNA aberrant alteration (i.e epigenomic profile) as well as changes in the tissue microenviroment, both into the niche and by systemic circulating factors. Strikingly, it has been suggested that aging-induced deterioration of stem cell functions may play a key role in the pathophysiology of the various aging-associated disorders. Therefore, understanding how resident stem cell age and affects near and distant tissues is fundamental. Here, we examine the current knowledge about aging mechanisms in several kinds of adult stem cells under physiological and pathological conditions and the principal aging-related changes in number, function and phenotype that determine the loss of tissue renewal properties. Furthermore, we examine the possible cell rejuvenation strategies. Stem cell rejuvenation may reverse the aging phenotype and the discovery of effective methods for inducing and differentiating pluripotent stem cells for cell replacement therapies could open up new possibilities for treating age-related diseases.
Keywords: Aging; Aging environment; Aging-associated disorders; Epigenetic changes; Rejuvenation; Self-renewal; Stem cells.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: None of the authors have any conflicts of interest.
Figures

References
-
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution . 1957;11:398–411.
-
- Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. - PubMed
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. - PubMed
-
- Zeng X. Human embryonic stem cells: mechanisms to escape replicative senescence? Stem Cell Rev. 2007;3:270–279. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

