Designing light-driven rotary molecular motors
- PMID: 34909140
- PMCID: PMC8612399
- DOI: 10.1039/d1sc04781g
Designing light-driven rotary molecular motors
Abstract
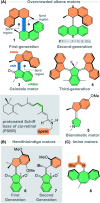
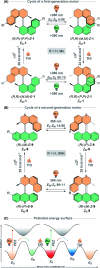
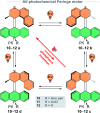
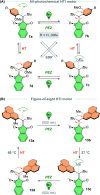
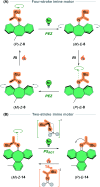
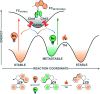
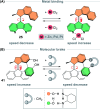
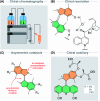
The ability to induce and amplify motion at the molecular scale has seen tremendous progress ranging from simple molecular rotors to responsive materials. In the two decades since the discovery of light-driven rotary molecular motors, the development of these molecules has been extensive; moving from the realm of molecular chemistry to integration into dynamic molecular systems. They have been identified as actuators holding great potential to precisely control the dynamics of nanoscale devices, but integrating molecular motors effectively into evermore complex artificial molecular machinery is not trivial. Maximising efficiency without compromising function requires conscious and judicious selection of the structures used. In this perspective, we focus on the key aspects of motor design and discuss how to manipulate these properties without impeding motor integrity. Herein, we describe these principles in the context of molecular rotary motors featuring a central double bond axle and emphasise the strengths and weaknesses of each design, providing a comprehensive evaluation of all artificial light-driven rotary motor scaffolds currently present in the literature. Based on this discussion, we will explore the trajectory of research into the field of molecular motors in the coming years, including challenges to be addressed, potential applications, and future prospects.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare there to be no conflicts of interest.
Figures






References
-
- Browne W. R. and Feringa B. L., Molecular Switches, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011
-
- Goodsell D. S., The Machinery of Life, Springer, New York, 2nd edn, 2009
Publication types
LinkOut - more resources
Full Text Sources

