Device-Associated Hospital-Acquired Infections: Does Active Surveillance With Bundle Care Offer a Pathway to Minimize Them?
- PMID: 34909294
- PMCID: PMC8651063
- DOI: 10.7759/cureus.19331
Device-Associated Hospital-Acquired Infections: Does Active Surveillance With Bundle Care Offer a Pathway to Minimize Them?
Abstract
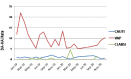
Background and objective The prevalence of hospital-acquired infections (HAIs) is underreported in developing nations due to a lack of systematic active surveillance. This study reports the burden of device-associated HAIs (DA-HAIs) based on two years of active surveillance with in situ bundle care in closed intensive care units (ICUs) of a tertiary care hospital. Materials and methods A prospective surveillance study was carried out in 140-bedded ICUs (2,100-bed hospital) of a tertiary care private medical college hospital. Daily active surveillance for catheter-associated urinary tract infection (CAUTI), ventilator-associated event (VAE), and central line-associated bloodstream infection (CLABSI) was done by trained infection control nurses (ICNs) along with quality champion nurses with HAI surveillance forms with bundle care auditing, which was attached to the case sheets of all patients on devices. The surveillance definitions of DA-HAIs were adapted from the Centers for Disease Control and Prevention (CDC)'s National Healthcare Safety Network (CDC-NHSN) 2017 surveillance criteria. Data were analyzed at the end of every month to generate the cumulative device-associated infection (DAI) rates and device utilization ratio (DUR). These data were compared with NHSN and International Nosocomial Infection Control Consortium (INICC) - India HAI rates and communicated to corresponding ICUs and also presented at the hospital infection control committee (HICC) meeting. Results The surveillance data were reported over 71,877 patient days during the study period. The DUR of urinary catheters, ventilator, and central line were 0.53, 0.16, and 0.22, respectively. CAUTI, VAE, and CLABSI rates were 0.97, 10.5, and 0.43 per 1,000 device days, respectively. Among 166 DA-HAIs reported, 182 pathogens were identified. Klebsiella pneumoniae was the most common organism isolated, accounting for 37.4% of all DA-HAI cases, followed by Acinetobacter baumanii (30.8%). Most of the Gram-negative organisms were carbapenem-resistant (153/175; 87.4%). Vancomycin resistance rate in Enterococcus was 28.5% (2/7). Conclusion DUR and CAUTI, VAE, CLABSI rates were less/on par with the benchmarks of INICC and CDC-NHSN in almost all ICUs of our tertiary care unit. Gram-negative pathogen with 87.4% carbapenem resistance worsened the scenario. Proper active surveillance with bundle care and training by ICNs made a significant difference in all DA-HAI rates, especially VAE, which decreased to 10.5 from 23.6 per 1,000 ventilator days. Sustained active surveillance of HAI and bundle care auditing by a trained infection prevention team with a stringent antibiotic policy are the need of the hour to combat DAIs.
Keywords: bundle care; central line; device associated infections; urinary catheter; ventilator.
Copyright © 2021, Ganesan et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Hospital-acquired infections: current trends and prevention. Boev C, Kiss E. Crit Care Nurs Clin North Am. 2017;29:51–65. - PubMed
-
- Does catheter-associated urinary tract infection increase mortality in critically ill patients? Clec'h C, Schwebel C, Français A, et al. Infect Control Hosp Epidemiol. 2007;28:1367–1373. - PubMed
-
- Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Mathur P, Tak V, Gunjiyal J, et al. Indian J Med Microbiol. 2015;33:51–62. - PubMed
-
- Device-associated infection rates in 20 cities of India, data summary for 2004-2013: findings of the International Nosocomial Infection Control Consortium. Mehta Y, Jaggi N, Rosenthal VD, et al. Infect Control Hosp Epidemiol. 2016;37:172–181. - PubMed
LinkOut - more resources
Full Text Sources
