US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives
- PMID: 34909579
- PMCID: PMC8664682
- DOI: 10.1093/abt/tbab027
US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives
Abstract
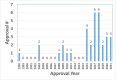
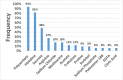
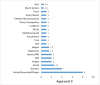
Thirty four (34) of the total US FDA approved 103 therapeutic antibody drugs, accounts for one third of the total approved mAbs, are formulated with high protein concentration (100 mg/mL or above) which are the focus of this article. The highest protein concentration of these approved mAbs is 200 mg/mL. The dominant administration route is subcutaneous (76%). Our analysis indicates that it may be rational to implement a platform formulation containing polysorbate, histidine and sucrose to accelerate high concentration formulation development for antibody drugs. Since 2015, the FDA approval numbers are significantly increased which account for 76% of the total approval numbers, i.e., 26 out of 34 highly concentrated antibodies. Thus, we believe that the high concentration formulations of antibody drugs will be the future trend of therapeutic antibody formulation development, regardless of the challenges of highly concentrated protein formulations.
Keywords: US FDA; administration route; approval; dosage; excipient; formulation; formulation composition; high concentration; lyophilization; pre-filled syringe; subcutaneous; therapeutic antibodies.
© The Author(s) 2021. Published by Oxford University Press on behalf of Antibody Therapeutics. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov 2021; 207: 491–5. - PubMed
-
- Shire, SJ, Shahrokh, Z, Liu, J. Challenges in the development of high protein concentration formulations. J Pharm Sci 2004; 93: 1390–402. - PubMed
-
- Sahin, E, Deshmukh, S. Challenges and considerations in development and manufacturing of high concentration biologics drug products. J Pharm Innov 2020; 15: 255–67.
-
- Lutz, H, Arias, J, Zou, Y. High concentration biotherapeutic formulation and ultrafiltration: part 1 pressure limits. Biotechnol Prog 2017; 33: 113–24. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
