Seeing through the Skin: Photoacoustic Tomography of Skin Vasculature and Beyond
- PMID: 34909735
- PMCID: PMC8659408
- DOI: 10.1016/j.xjidi.2021.100039
Seeing through the Skin: Photoacoustic Tomography of Skin Vasculature and Beyond
Abstract
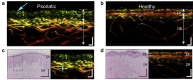
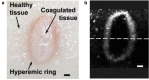
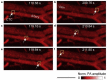
Skin diseases are the most common human diseases and manifest in distinct structural and functional changes to skin tissue components such as basal cells, vasculature, and pigmentation. Although biopsy is the standard practice for skin disease diagnosis, it is not sufficient to provide in vivo status of the skin and highly depends on the timing of diagnosis. Noninvasive imaging technologies that can provide structural and functional tissue information in real time would be invaluable for skin disease diagnosis and treatment evaluation. Among the modern medical imaging technologies, photoacoustic (PA) tomography (PAT) shows great promise as an emerging optical imaging modality with high spatial resolution, high imaging speed, deep penetration depth, rich contrast, and inherent sensitivity to functional and molecular information. Over the last decade, PAT has undergone an explosion in technical development and biomedical applications. Particularly, PAT has attracted increasing attention in skin disease diagnosis, providing structural, functional, metabolic, molecular, and histological information. In this concise review, we introduce the principles and imaging capability of various PA skin imaging technologies. We highlight the representative applications in the past decade with a focus on imaging skin vasculature and melanoma. We also envision the critical technical developments necessary to further accelerate the translation of PAT technologies to fundamental skin research and clinical impacts.
Keywords: ACD, allergy contact dermatitis; AR-PAM, acoustic-resolution photoacoustic microscopy; CSC, cryogen spray cooling; CSVV, cutaneous small-vessel vasculitis; CTC, circulating tumor cell; FDA, Food and Drug Administration; NIR, near-infrared; OR-PAM, optical-resolution photoacoustic microscopy; PA, photoacoustic; PACT, photoacoustic computed tomography; PAM, photoacoustic microscopy; PAT, photoacoustic tomography; PWS, port-wine stain; RSOM, raster-scan optoacoustic mesoscopy; THb, total hemoglobin concentration; sO2, oxygen saturation of hemoglobin.
© 2021 The Authors.
Figures
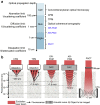

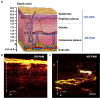
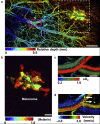
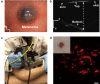
References
-
- Aguirre J., Schwarz M., Garzorz N., Omar M., Buehler A., Eyerich K., et al. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat Biomed Eng. 2017;1:1–8.
-
- Aizawa K., Sato S., Saitoh D., Ashida H., Obara M. Photoacoustic monitoring of burn healing process in rats. J Biomed Opt. 2008;13:064020. - PubMed
-
- Argenziano G., Zalaudek I., Corona R., Sera F., Cicale L., Petrillo G., et al. Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol. 2004;140:1485–1489. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

