SARS-CoV-2 spike protein harnesses SNX27-mediated endocytic recycling pathway
- PMID: 34909756
- PMCID: PMC8661858
- DOI: 10.1002/mco2.92
SARS-CoV-2 spike protein harnesses SNX27-mediated endocytic recycling pathway
Abstract
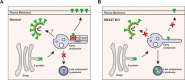
SARS-CoV-2 is an enveloped positive-sense RNA virus that depends on host factors for all stages of its life. Membrane receptor ACE2 is a well-established factor for SARS-CoV-2 docking. In addition to ACE2, whole-genome genetic screens have identified additional proteins, such as endosomal trafficking regulators SNX27 and retromer, as key host factors required for SARS-CoV-2 infection. However, it is poorly understood how SARS-CoV-2 utilize host endocytic transport pathways to produce productive infection. Here, we report that SNX27 interacts with the SARS-CoV-2 spike (S) protein to facilitate S protein surface expression. Interestingly, S protein binds to the PDZ domain of SNX27, although it does not contain a PDZ-binding motif (PDZbm). Either abrogation of the SNX27 PDZ domain or S protein "MTSC" motif, which is critical for SNX27 binding, decreases surface expression of S protein and viral production. Collectively, our study highlights a novel approach utilized by SARS-CoV-2 to facilitate virion trafficking to establish virus infection.
Keywords: S protein; SARS‐CoV‐2; endocytic trafficking; endosome; host–pathogen interaction.
© 2021 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
