Transcriptional Pathology Evolves over Time in Rat Hippocampus after Lateral Fluid Percussion Traumatic Brain Injury
- PMID: 34909768
- PMCID: PMC8667199
- DOI: 10.1089/neur.2021.0021
Transcriptional Pathology Evolves over Time in Rat Hippocampus after Lateral Fluid Percussion Traumatic Brain Injury
Abstract
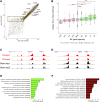
Traumatic brain injury (TBI) causes acute and lasting impacts on the brain, driving pathology along anatomical, cellular, and behavioral dimensions. Rodent models offer an opportunity to study the temporal progression of disease from injury to recovery. Transcriptomic and epigenomic analysis were applied to evaluate gene expression in ipsilateral hippocampus at 1 and 14 days after sham (n = 2 and 4, respectively per time point) and moderate lateral fluid percussion injury (n = 4 per time point). This enabled the identification of dynamic changes and differential gene expression (differentially expressed genes; DEGs) modules linked to underlying epigenetic response. We observed acute signatures associated with cell death, astrocytosis, and neurotransmission that largely recovered by 2 weeks. Inflammation and immune signatures segregated into upregulated modules with distinct expression trajectories and functions. Whereas most down-regulated genes recovered by 14 days, two modules with delayed and persistent changes were associated with cholesterol metabolism, amyloid beta clearance, and neurodegeneration. Differential expression was paralleled by changes in histone H3 lysine residue 4 trimethylation at the promoters of DEGs at 1 day post-TBI, with the strongest changes observed for inflammation and immune response genes. These results demonstrate how integrated genomics analysis in the pre-clinical setting has the potential to identify stage-specific biomarkers for injury and/or recovery. Though limited in scope here, our general strategy has the potential to capture pathological signatures over time and evaluate treatment efficacy at the systems level.
Keywords: TBI; differential expression; longitudinal; neurodegeneration; rat.
© Rinaldo Catta-Preta et al., 2021; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Centers for Disease Control and Prevention. (2019). Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. Centers for Disease Control and Prevention: Atlanta, GA.
-
- Defense and Veterans Brain Injury Center. (2019). DoD Numbers for Traumatic Brain Injury Worldwide—Totals 2019 Q1-Q4 (https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTo...). [cited 2020 Jun 25 ]. https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTo... (Last accessed April 16, 2019).
-
- Masel, B.E., and DeWitt, D.S. (2010). Traumatic brain injury: a disease process, not an event. J. Neurotrauma 27, 1529–1540. - PubMed
-
- Crane, P.K., Gibbons, L.E., Dams-O'Connor, K., Trittschuh, E., Leverenz, J.B., Keene, C.D., Sonnen, J., Montine, T.J., Bennett, D.A., Leurgans, S., Schneider, J.A., and Larson, E.B. (2016). Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 73, 1062–1069. - PMC - PubMed
-
- Chauhan, N.B. (2014). Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 32, 337–365. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
