This is a preprint.
An Open One-Step RT-qPCR for SARS-CoV-2 detection
- PMID: 34909786
- PMCID: PMC8669853
- DOI: 10.1101/2021.11.29.21267000
An Open One-Step RT-qPCR for SARS-CoV-2 detection
Abstract
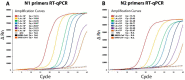
The COVID-19 pandemic has resulted in millions of deaths globally, and while several diagnostic systems were proposed, real-time reverse transcription polymerase chain reaction (RT-PCR) remains the gold standard. However, diagnostic reagents, including enzymes used in RT-PCR, are subject to centralized production models and intellectual property restrictions, which present a challenge for less developed countries. With the aim of generating a standardized One-Step open RT-qPCR protocol to detect SARS-CoV-2 RNA in clinical samples, we purified and tested recombinant enzymes and a non-proprietary buffer. The protocol utilized M-MLV RT and Taq DNA pol enzymes to perform a Taqman probe-based assay. Synthetic RNA samples were used to validate the One-Step RT-qPCR components, and the kit showed comparable sensitivity to approved commercial kits. The One-Step RT-qPCR was then tested on clinical samples and demonstrated similar performance to commercial kits in terms of positive and negative calls. This study represents a proof of concept for an open approach to developing diagnostic kits for viral infections and diseases, which could provide a cost-effective and accessible solution for less developed countries.
Keywords: Diagnostic system; One-Step RT-PCR; Open Source; Recombinant enzymes; SARS-CoV-2.
Conflict of interest statement
Competing interests The authors have declared that no competing interests exist.
Figures

References
-
- (WHO), W.H.O. Weekly epidemiological update on COVID-19 – 1 June 2022. 2022. [cited 2022 June, 2022]; Available from: http://www.who.int/publications/m/item/weekly-epidemiological-update-on-....
-
- (WHO), W.H.O. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. 2022. [cited 2022 June, 2022]; Available from: http://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
