Association of Complement and MAPK Activation With SARS-CoV-2-Associated Myocardial Inflammation
- PMID: 34910083
- PMCID: PMC8674808
- DOI: 10.1001/jamacardio.2021.5133
Association of Complement and MAPK Activation With SARS-CoV-2-Associated Myocardial Inflammation
Abstract
Importance: Myocardial injury is a common feature of patients with SARS-CoV-2 infection. However, the cardiac inflammatory processes associated with SARS-CoV-2 infection are not completely understood.
Objective: To investigate the inflammatory cardiac phenotype associated with SARS-CoV-2 infection compared with viral myocarditis, immune-mediated myocarditis, and noninflammatory cardiomyopathy by integrating histologic, transcriptomic, and proteomic profiling.
Design, setting, and participants: This case series was a cooperative study between the Ludwig Maximilian University Hospital Munich and the Cardiopathology Referral Center at the University of Tübingen in Germany. A cohort of 19 patients with suspected myocarditis was examined; of those, 5 patients were hospitalized with SARS-CoV-2 infection between March and May 2020. Cardiac tissue specimens from those 5 patients were compared with specimens from 5 patients with immune-mediated myocarditis, 4 patients with non-SARS-CoV-2 viral myocarditis, and 5 patients with noninflammatory cardiomyopathy, collected from January to August 2019.
Exposures: Endomyocardial biopsy.
Main outcomes and measures: The inflammatory cardiac phenotypes were measured by immunohistologic analysis, RNA exome capture sequencing, and mass spectrometry-based proteomic analysis of endomyocardial biopsy specimens.
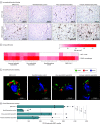
Results: Among 19 participants, the median age was 58 years (range, 37-76 years), and 15 individuals (79%) were male. Data on race and ethnicity were not collected. The abundance of CD163+ macrophages was generally higher in the cardiac tissue of patients with myocarditis, whereas lymphocyte counts were lower in the tissue of patients with SARS-CoV-2 infection vs patients with non-SARS-CoV-2 virus-associated and immune-mediated myocarditis. Among those with SARS-CoV-2 infection, components of the complement cascade, including C1q subunits (transcriptomic analysis: 2.5-fold to 3.6-fold increase; proteomic analysis: 2.0-fold to 3.4-fold increase) and serine/cysteine proteinase inhibitor clade G member 1 (transcriptomic analysis: 1.7-fold increase; proteomic analysis: 2.6-fold increase), belonged to the most commonly upregulated transcripts and differentially abundant proteins. In cardiac macrophages, the abundance of C1q was highest in SARS-CoV-2 infection. Assessment of important signaling cascades identified an upregulation of the serine/threonine mitogen-activated protein kinase pathways.
Conclusions and relevance: This case series found that the cardiac immune signature varied in inflammatory conditions with different etiologic characteristics. Future studies are needed to examine the role of these immune pathways in myocardial inflammation.
Conflict of interest statement
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

