Incidence of Herpes Simplex Virus Type 2 Infection Among African Women Using Depot Medroxyprogesterone Acetate, a Copper Intrauterine Device, or a Levonorgestrel Implant for Contraception: A Nested Randomized Trial
- PMID: 34910143
- PMCID: PMC9464069
- DOI: 10.1093/cid/ciab1027
Incidence of Herpes Simplex Virus Type 2 Infection Among African Women Using Depot Medroxyprogesterone Acetate, a Copper Intrauterine Device, or a Levonorgestrel Implant for Contraception: A Nested Randomized Trial
Erratum in
-
Correction to: Incidence of Herpes Simplex Virus Type 2 Infection Among African Women Using Depot Medroxyprogesterone Acetate, a Copper Intrauterine Device, or a Levonorgestrel Implant for Contraception: A Nested Randomized Trial.Clin Infect Dis. 2023 Mar 4;76(5):972. doi: 10.1093/cid/ciac309. Clin Infect Dis. 2023. PMID: 36689296 Free PMC article. No abstract available.
Abstract
Background: Globally, women have higher herpes simplex virus type 2 (HSV-2) prevalence than men; data from observational studies suggest a possible association of HSV-2 acquisition with use of intramuscular depot medroxyprogesterone acetate (DMPA-IM).
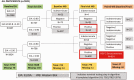
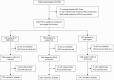
Methods: Within a randomized trial of the effect of 3 contraceptive methods-DMPA-IM, a copper intrauterine device (IUD), and a levonorgestrel (LNG) implant-on human immunodeficiency virus (HIV) acquisition, we assessed HSV-2 acquisition. HSV-2 and HIV seronegative women, aged 16-35 years, and seeking effective contraception were followed for 12-18 months at 12 sites in Eswatini, Kenya, South Africa, and Zambia from 2015 to 2018. HSV-2 serologic testing was done at enrollment and final study visits. Intention-to-treat analysis using Poisson regression with robust standard errors compared HSV-2 incidence by contraceptive method.
Results: At baseline, 4062 randomized women were HSV-2 seronegative, of whom 3898 (96.0%) had a conclusive HSV-2 result at their final study visit. Of these, 614 (15.8%) acquired HSV-2, at an incidence of 12.4/100 person-years (p-y): 10.9/100 p-y among women assigned DMPA-IM, 13.7/100 p-y the copper IUD, and 12.7/100 p-y the LNG implant. Incidence rate ratios (IRR) for HSV-2 acquisition were 0.80 (95% confidence interval [CI], .65-.97) for DMPA-IM compared with copper IUD, 0.86 (95% CI, .71-1.05) for DMPA-IM compared with LNG implant, and 1.08 (95% CI, .89-1.30) for copper IUD compared with LNG implant. HSV-2 acquisition risk was significantly increased among women who also acquired HIV during follow-up (IRR 3.55; 95% CI, 2.78-4.48).
Conclusions: In a randomized trial, we found no association between HSV-2 acquisition and use of 3 contraceptive methods.
Trial registration: ClinicalTrials.gov number NCT02550067.
Keywords: Africa; HIV; contraception; herpes simplex virus type 2 (HSV-2); women.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. A. B. reports support for the present manuscript from the WHO Department of RH. D. D. reports support for the present manuscript from National Institute of Mental Health paid to the institution and grants or contracts outside of the conduct of the study from the National Institute of Allergy and Infectious Diseases paid to the institution. J. B. reports grants or contracts outside of the conduct of the study from National Institutes of Health paid to the institution; personal consulting fees from Gilead, Janssen, and Merck; employee/stock/options from Gilead; donation of study medication from Gilead and IPM; and employee outside of the submitted work for Gilead Sciences. R. S. reports direct travel reimbursements and travel scholarships for Conference on Retroviruses and Opportunistic Infections, AIDS, Infectious Diseases Society for Obstetrics and Gynecology, and International Society for Pharmacoepidemiology conferences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001; 357:1149–53. - PubMed