Infection-induced chromatin modifications facilitate translocation of herpes simplex virus capsids to the inner nuclear membrane
- PMID: 34910768
- PMCID: PMC8673650
- DOI: 10.1371/journal.ppat.1010132
Infection-induced chromatin modifications facilitate translocation of herpes simplex virus capsids to the inner nuclear membrane
Abstract
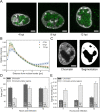
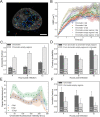
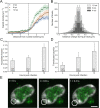
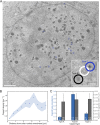
Herpes simplex virus capsids are assembled and packaged in the nucleus and move by diffusion through the nucleoplasm to the nuclear envelope for egress. Analyzing their motion provides conclusions not only on capsid transport but also on the properties of the nuclear environment during infection. We utilized live-cell imaging and single-particle tracking to characterize capsid motion relative to the host chromatin. The data indicate that as the chromatin was marginalized toward the nuclear envelope it presented a restrictive barrier to the capsids. However, later in infection this barrier became more permissive and the probability of capsids to enter the chromatin increased. Thus, although chromatin marginalization initially restricted capsid transport to the nuclear envelope, a structural reorganization of the chromatin counteracted that to promote capsid transport later. Analyses of capsid motion revealed that it was subdiffusive, and that the diffusion coefficients were lower in the chromatin than in regions lacking chromatin. In addition, the diffusion coefficient in both regions increased during infection. Throughout the infection, the capsids were never enriched at the nuclear envelope, which suggests that instead of nuclear export the transport through the chromatin is the rate-limiting step for the nuclear egress of capsids. This provides motivation for further studies by validating the importance of intranuclear transport to the life cycle of HSV-1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Ojala PM, Sodeik B, Ebersold MW, Kutay U, Helenius A. Herpes Simplex Virus Type 1 Entry into Host Cells: Reconstitution of Capsid Binding and Uncoating at the Nuclear Pore Complex In Vitro. Molecular and Cellular Biology. 2000;20(13):4922–31. doi: 10.1128/MCB.20.13.4922-4931.2000 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

