The onset of circulation triggers a metabolic switch required for endothelial to hematopoietic transition
- PMID: 34910918
- PMCID: PMC8692754
- DOI: 10.1016/j.celrep.2021.110103
The onset of circulation triggers a metabolic switch required for endothelial to hematopoietic transition
Abstract
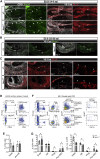
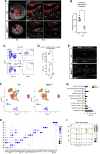
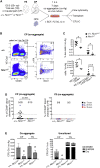
Hematopoietic stem cells (HSCs) emerge during development from the vascular wall of the main embryonic arteries. The onset of circulation triggers several processes that provide critical external factors for HSC generation. Nevertheless, it is not fully understood how and when the onset of circulation affects HSC emergence. Here we show that in Ncx1-/- mouse embryos devoid of circulation the HSC lineage develops until the phenotypic pro-HSC stage. However, these cells reside in an abnormal microenvironment, fail to activate the hematopoietic program downstream of Runx1, and are functionally impaired. Single-cell transcriptomics shows that during the endothelial-to-hematopoietic transition, Ncx1-/- cells fail to undergo a glycolysis to oxidative phosphorylation metabolic switch present in wild-type cells. Interestingly, experimental activation of glycolysis results in decreased intraembryonic hematopoiesis. Our results suggest that the onset of circulation triggers metabolic changes that allow HSC generation to proceed.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Baratchi S., Khoshmanesh K., Woodman O.L., Potocnik S., Peter K., McIntyre P. Molecular sensors of blood flow in endothelial cells. Trends in Molecular Medicine. 2017;23:850–868. 868. - PubMed
-
- Batsivari A., Rybtsov S., Souilhol C., Binagui-Casas A., Hills D., Zhao S., Travers P., Medvinsky A. Understanding hematopoietic stem cell development through functional correlation of their proliferative status with the intra-aortic cluster architecture. Stem Cell Reports. 2017;8:1549–1562. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016058/CA/NCI NIH HHS/United States
- U24 CA232979/CA/NCI NIH HHS/United States
- MC_UU_12009/2/MRC_/Medical Research Council/United Kingdom
- G0902418/MRC_/Medical Research Council/United Kingdom
- 18974/CRUK_/Cancer Research UK/United Kingdom
- G0801073/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/5/MRC_/Medical Research Council/United Kingdom
- G0501838/MRC_/Medical Research Council/United Kingdom
- MC_UU_00016/5/MRC_/Medical Research Council/United Kingdom
- P30 CA016056/CA/NCI NIH HHS/United States
- MC_UU_00016/2/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

