Elevated Wnt2 and Wnt4 activate NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 following myocardial infarction
- PMID: 34911029
- PMCID: PMC8669316
- DOI: 10.1016/j.ebiom.2021.103745
Elevated Wnt2 and Wnt4 activate NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 following myocardial infarction
Abstract
Background: Acute myocardial infarction (AMI)-induced excessive myocardial fibrosis exaggerates cardiac dysfunction. However, serum Wnt2 or Wnt4 level in AMI patients, and the roles in cardiac fibrosis are largely unkown.
Methods: AMI and non-AMI patients were enrolled to examine serum Wnt2 and Wnt4 levels by ELISA analysis. The AMI patients were followed-up for one year. MI mouse model was built by ligation of left anterior descending branch (LAD).
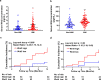
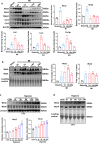
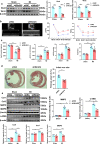
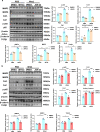
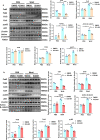
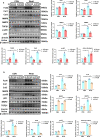
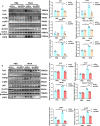
Findings: Serum Wnt2 or Wnt4 level was increased in patients with AMI, and the elevated Wnt2 and Wnt4 were correlated to adverse outcome of these patients. Knockdown of Wnt2 and Wnt4 significantly attenuated myocardial remodeling and cardiac dysfunction following experimental MI. In vitro, hypoxia enhanced the secretion and expression of Wnt2 and Wnt4 in neonatal rat cardiac myocytes (NRCMs) or fibroblasts (NRCFs). Mechanistically, the elevated Wnt2 or Wnt4 activated β-catenin /NF-κB signaling to promote pro-fibrotic effects in cultured NRCFs. In addition, Wnt2 or Wnt4 upregulated the expression of these Wnt co-receptors, frizzled (Fzd) 2, Fzd4 and (low-density lipoprotein receptor-related protein 6 (LRP6). Further analysis revealed that Wnt2 or Wnt4 activated β-catenin /NF-κB by the co-operation of Fzd4 or Fzd2 and LRP6 signaling, respectively.
Interpretation: Elevated Wnt2 and Wnt4 activate β-catenin/NF-κB signaling to promote cardiac fibrosis by cooperation of Fzd4/2 and LRP6 in fibroblasts, which contributes to adverse outcome of patients with AMI, suggesting that systemic inhibition of Wnt2 and Wnt4 may improve cardiac dysfunction after MI.
Keywords: Cardiac fibrosis; Frizzled; Myocardial infarction; NF-κB; Wnt.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures



Similar articles
-
β-Catenin-dependent pathway activation by both promiscuous "canonical" WNT3a-, and specific "noncanonical" WNT4- and WNT5a-FZD receptor combinations with strong differences in LRP5 and LRP6 dependency.Cell Signal. 2014 Feb;26(2):260-7. doi: 10.1016/j.cellsig.2013.11.021. Epub 2013 Nov 21. Cell Signal. 2014. PMID: 24269653
-
Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening.Circ Res. 2011 Feb 18;108(4):427-36. doi: 10.1161/CIRCRESAHA.110.233999. Epub 2010 Dec 30. Circ Res. 2011. PMID: 21193738
-
Identification of WNT/beta-CATENIN signaling pathway components in human cumulus cells.Mol Hum Reprod. 2009 Jan;15(1):11-7. doi: 10.1093/molehr/gan070. Epub 2008 Nov 26. Mol Hum Reprod. 2009. PMID: 19038973
-
Qiliqiangxin Attenuates Cardiac Remodeling via Inhibition of TGF-β1/Smad3 and NF-κB Signaling Pathways in a Rat Model of Myocardial Infarction.Cell Physiol Biochem. 2018;45(5):1797-1806. doi: 10.1159/000487871. Epub 2018 Feb 28. Cell Physiol Biochem. 2018. PMID: 29510381
-
WNT4 Balances Development vs Disease in Gynecologic Tissues and Women's Health.Endocrinology. 2021 Jul 1;162(7):bqab093. doi: 10.1210/endocr/bqab093. Endocrinology. 2021. PMID: 33963381 Free PMC article. Review.
Cited by
-
Transcriptome Analysis of Myocardial Ischemic-Hypoxic Injury in Rats and Hypoxic H9C2 Cells.ESC Heart Fail. 2024 Dec;11(6):3775-3795. doi: 10.1002/ehf2.14903. Epub 2024 Jul 15. ESC Heart Fail. 2024. PMID: 39010664 Free PMC article.
-
The role of β-catenin in cardiac diseases.Front Pharmacol. 2023 Mar 22;14:1157043. doi: 10.3389/fphar.2023.1157043. eCollection 2023. Front Pharmacol. 2023. PMID: 37033656 Free PMC article. Review.
-
Silencing hsa_circ_0049271 attenuates hypoxia-reoxygenation (H/R)-induced myocardial cell injury via the miR-17-3p/FZD4 signaling axis.Ann Transl Med. 2023 Jan 31;11(2):99. doi: 10.21037/atm-22-6331. Ann Transl Med. 2023. PMID: 36819541 Free PMC article.
-
Complex regulation of cardiac fibrosis: insights from immune cells and signaling pathways.J Transl Med. 2025 Feb 28;23(1):242. doi: 10.1186/s12967-025-06260-5. J Transl Med. 2025. PMID: 40022104 Free PMC article. Review.
-
lncRNA LOC100911717-targeting GAP43-mediated sympathetic remodeling after myocardial infarction in rats.Front Cardiovasc Med. 2023 Jan 6;9:1019435. doi: 10.3389/fcvm.2022.1019435. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36684596 Free PMC article.
References
-
- Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. - PubMed
-
- Seropian I.M., Toldo S., Van Tassell B.W., Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63(16):1593–1603. - PubMed
-
- van den Borne S.W., Diez J., Blankesteijn W.M., Verjans J., Hofstra L., Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7(1):30–37. - PubMed
-
- Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. The role of cardiac fibroblasts in post-myocardial heart tissue repair. Exp Mol Pathol. 2016;101(2):231–240. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

