Cell-extracellular matrix dynamics
- PMID: 34911051
- PMCID: PMC8855216
- DOI: 10.1088/1478-3975/ac4390
Cell-extracellular matrix dynamics
Abstract
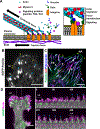
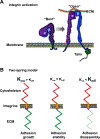
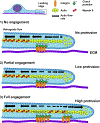
The sites of interaction between a cell and its surrounding microenvironment serve as dynamic signaling hubs that regulate cellular adaptations during developmental processes, immune functions, wound healing, cell migration, cancer invasion and metastasis, as well as in many other disease states. For most cell types, these interactions are established by integrin receptors binding directly to extracellular matrix proteins, such as the numerous collagens or fibronectin. For the cell, these points of contact provide vital cues by sampling environmental conditions, both chemical and physical. The overall regulation of this dynamic interaction involves both extracellular and intracellular components and can be highly variable. In this review, we highlight recent advances and hypotheses about the mechanisms and regulation of cell-ECM interactions, from the molecular to the tissue level, with a particular focus on cell migration. We then explore how cancer cell invasion and metastasis are deeply rooted in altered regulation of this vital interaction.
Keywords: cell migration; dynamics; extracellular matrix; focal adhesions.
Creative Commons Attribution license.
Figures


References
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
