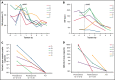
SARS-CoV-2-neutralizing antibody treatment in patients with COVID-19 and immunodeficiency due to B-cell non-Hodgkin lymphoma
- PMID: 34911078
- PMCID: PMC8683250
- DOI: 10.1182/bloodadvances.2021006655
SARS-CoV-2-neutralizing antibody treatment in patients with COVID-19 and immunodeficiency due to B-cell non-Hodgkin lymphoma
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

