European LeukemiaNet 2017 risk stratification for acute myeloid leukemia: validation in a risk-adapted protocol
- PMID: 34911079
- PMCID: PMC8864653
- DOI: 10.1182/bloodadvances.2021005585
European LeukemiaNet 2017 risk stratification for acute myeloid leukemia: validation in a risk-adapted protocol
Abstract
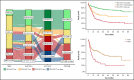
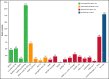
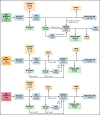
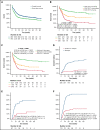
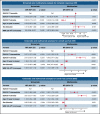
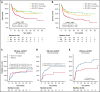
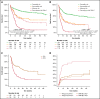
The 2017 European LeukemiaNet (ELN 2017) guidelines for the diagnosis and management of acute myeloid leukemia (AML) have become fundamental guidelines to assess the prognosis and postremission therapy of patients. However, they have been retrospectively validated in few studies with patients included in different treatment protocols. We analyzed 861 patients included in the Cooperativo Para el Estudio y Tratamiento de las Leucemias Agudas y Mielodisplasias-12 risk-adapted protocol, which indicates cytarabine-based consolidation for patients allocated to the ELN 2017 favorable-risk group, whereas it recommends allogeneic stem cell transplantation (alloSCT) as a postremission strategy for the ELN 2017 intermediate- and adverse-risk groups. We retrospectively classified patients according to the ELN 2017, with 327 (48%), 109 (16%), and 245 (36%) patients allocated to the favorable-, intermediate-, and adverse-risk group, respectively. The 2- and 5-year overall survival (OS) rates were 77% and 70% for favorable-risk patients, 52% and 46% for intermediate-risk patients, and 33% and 23% for adverse-risk patients, respectively. Furthermore, we identified a subgroup of patients within the adverse group (inv(3)/t(3;3), complex karyotype, and/or TP53 mutation/17p abnormality) with a particularly poor outcome, with a 2-year OS of 15%. Our study validates the ELN 2017 risk stratification in a large cohort of patients treated with an ELN-2017 risk-adapted protocol based on alloSCT after remission for nonfavorable ELN subgroups and identifies a genetic subset with a very poor outcome that warrants investigation of novel strategies.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. - PubMed
-
- Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642-4649. - PubMed
-
- Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet . Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. - PubMed
-
- Röllig C, Bornhäuser M, Thiede C, et al. . Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758-2765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

