Dopaminergic pathways and resting-state functional connectivity in Parkinson's disease with freezing of gait
- PMID: 34911202
- PMCID: PMC8645514
- DOI: 10.1016/j.nicl.2021.102899
Dopaminergic pathways and resting-state functional connectivity in Parkinson's disease with freezing of gait
Abstract
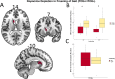
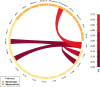
Freezing of gait is a common phenomenon of advanced Parkinson's disease. Besides locomotor function per se, a role of cognitive deficits has been suggested. Limited evidence of associated dopaminergic deficits points to caudatal denervation. Further, altered functional connectivity within resting-state networks with importance for cognitive functions has been described in freezers. A potential pathophysiological link between both imaging findings has not yet been addressed. The current study sought to investigate the association between dopaminergic pathway dysintegrity and functional dysconnectivity in relation to FOG severity and cognitive performance in a well-characterized PD cohort undergoing high-resolution 6-[18F]fluoro-L-Dopa PET and functional MRI. The freezing of gait questionnaire was applied to categorize patients (n = 59) into freezers and non-freezers. A voxel-wise group comparison of 6-[18F]fluoro-L-Dopa PET scans with focus on striatum was performed between both well-matched and neuropsychologically characterized patient groups. Seed-to-voxel resting-state functional connectivity maps of the resulting dopamine depleted structures and dopaminergic midbrain regions were created and compared between both groups. For a direct between-group comparison of dopaminergic pathway integrity, a molecular connectivity approach was conducted on 6-[18F]fluoro-L-Dopa scans. With respect to striatal regions, freezers showed significant dopaminergic deficits in the left caudate nucleus, which exhibited altered functional connectivity with regions of the visual network. Regarding midbrain structures, the bilateral ventral tegmental area showed altered functional coupling to regions of the default mode network. An explorative examination of the integrity of dopaminergic pathways by molecular connectivity analysis revealed freezing-associated impairments in mesolimbic and mesocortical pathways. This study represents the first characterization of a link between dopaminergic pathway dysintegrity and altered functional connectivity in Parkinson's disease with freezing of gait and hints at a specific involvement of striatocortical and mesocorticolimbic pathways in freezers.
Keywords: Freezing of gait; Functional connectivity; Molecular connectivity; Multimodal imaging; PET.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Moore O., Peretz C., Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov. Disord. 2007;22(15):2192–2195. - PubMed
-
- Spildooren J., Vercruysse S., Desloovere K., Vandenberghe W., Kerckhofs E., Nieuwboer A. Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov. Disord. 2010;25(15):2563–2570. - PubMed
-
- Peterson, D.S., King, L.A., Cohen, R.G., Horak, F.B. 2016. Cognitive contributions to freezing of gait in parkinson disease: Implications for physical rehabilitation. Phys. Ther. [online serial]. American Physical Therapy Association; 96, 659–670. Accessed at: /pmc/articles/PMC4858659/?report=abstract. Accessed August 18, 2020. - PMC - PubMed
-
- Giladi N., Hausdorff J.M. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J. Neurol. Sci. 2006;248(1-2):173–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

