Cell-sized, supported artificial membranes (pseudocytes): response of precursor cytotoxic T lymphocytes to class I MHC proteins
- PMID: 3491134
- PMCID: PMC10759930
Cell-sized, supported artificial membranes (pseudocytes): response of precursor cytotoxic T lymphocytes to class I MHC proteins
Abstract
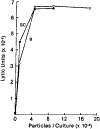
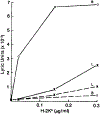
Novel cell-sized, supported artificial membranes bearing class I antigens have been prepared by a simple dialysis procedure and then used to study the requirements for antigen recognition by precursor cytotoxic T lymphocytes (CTL). The membranes were made by mixing lipid, H-2 antigen, and C18 alkylated 5 microns silica beads in deoxycholate, and dialyzing to remove the detergent. The H-2 antigen-bearing, cell-sized beads, termed pseudocytes (artificial cells), were able to simulate generation of secondary CTL responses with the same specificity as alloantigen-bearing spleen cells. Comparative analyses demonstrated that the size of an antigen-bearing structure, and thus its potential for multivalent interaction, was a critical determinant of effectiveness of antigen recognition, and showed that H-2 antigen was recognized as effectively on cell-sized beads as on allogeneic spleen cells. Generation of a response to antigen on the cell-sized beads was completely dependent on addition of lymphokines to the cultures. Thus, unlike liposomes, H-2 antigen on beads was not available to accessory cells for stimulation of Ia-dependent production of lymphokines by T helper cells. These results, as well as direct observations by microscopy, strongly indicate that antigen is recognized on the surface of the beads. Despite effective stimulation of secondary CTL responses, antigen on beads was completely inactive in stimulating a primary CTL response by naive spleen cells. The results of mixing experiments by using beads and alloantigen-bearing cells or plasma membrane vesicles indicate that the lack of a primary response may result from a requirement for a soluble factor(s) that is not needed for generation of secondary responses. The unique advantages of cell-size supported membranes for studying antigen recognition by T cells are discussed. The beads can be handled and used like antigen-bearing cells in functional assays, while possessing well-defined, readily varied, and easily quantitated composition.
Figures



Similar articles
-
Cytotoxic T cell activation by class I protein on cell-size artificial membranes: antigen density and Lyt-2/3 function.J Immunol. 1987 Apr 1;138(7):2034-43. J Immunol. 1987. PMID: 2951431
-
The requirements for antigen multivalency in class I antigen recognition and triggering of primed precursor cytolytic T lymphocytes.J Immunol. 1986 Apr 15;136(8):2816-25. J Immunol. 1986. PMID: 3082973
-
The ability of Ia and H-2Kk-bearing membranes to replace the antigen-presenting cell in an H-2Kk allogeneic cytotoxic T cell response.Eur J Immunol. 1985 Oct;15(10):1013-8. doi: 10.1002/eji.1830151010. Eur J Immunol. 1985. PMID: 2932336
-
Cell surface structures involved in T cell activation.Immunol Rev. 1984 Oct;81:65-94. doi: 10.1111/j.1600-065x.1984.tb01105.x. Immunol Rev. 1984. PMID: 6239820 Review.
-
Molecular interactions in the activation of effector and precursor cytotoxic T lymphocytes.Immunol Rev. 1995 Aug;146:177-210. doi: 10.1111/j.1600-065x.1995.tb00689.x. Immunol Rev. 1995. PMID: 7493753 Review.
Cited by
-
Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds.Nat Protoc. 2020 Mar;15(3):773-798. doi: 10.1038/s41596-019-0249-0. Epub 2020 Jan 13. Nat Protoc. 2020. PMID: 31932772
-
Bioengineering of Artificial Antigen Presenting Cells and Lymphoid Organs.Theranostics. 2017 Aug 17;7(14):3504-3516. doi: 10.7150/thno.19017. eCollection 2017. Theranostics. 2017. PMID: 28912891 Free PMC article. Review.
-
Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells.Trends Biotechnol. 2014 Sep;32(9):456-65. doi: 10.1016/j.tibtech.2014.06.007. Epub 2014 Jul 3. Trends Biotechnol. 2014. PMID: 24998519 Free PMC article. Review.
-
Cytoskeletal function in CD8- and T cell receptor-mediated interaction of cytotoxic T lymphocytes with class I protein.J Exp Med. 1991 Jan 1;173(1):241-9. doi: 10.1084/jem.173.1.241. J Exp Med. 1991. PMID: 1898662 Free PMC article.
-
Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium.Proc Natl Acad Sci U S A. 1990 Jul;87(13):5183-7. doi: 10.1073/pnas.87.13.5183. Proc Natl Acad Sci U S A. 1990. PMID: 2164225 Free PMC article.
References
-
- Benacerraf B. 1981. Role of MHC gene products in immune regulation. Science 212:1229. - PubMed
-
- Klein J. 1979. The major histocompatibility complex of the mouse. Science 203:516. - PubMed
-
- Klein J, Juretic A, Baxenis CN, and Nagy ZA. 1981. A traditional and a new version of the mouse H-2 complex. Nature 291:455. - PubMed
-
- Herrmann SH, Weinberger O, Burakoff SJ, and Mescher MF. 1982. Analysis of the two-signal requirement for precursor cytolytic T lymphocyte activation using H-2Kk liposomes. J. Immunol 128:1968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
