Advances in the design and development of SARS-CoV-2 vaccines
- PMID: 34911569
- PMCID: PMC8674100
- DOI: 10.1186/s40779-021-00360-1
Advances in the design and development of SARS-CoV-2 vaccines
Abstract
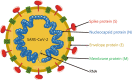
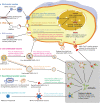
Since the end of 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide. The RNA genome of SARS-CoV-2, which is highly infectious and prone to rapid mutation, encodes both structural and nonstructural proteins. Vaccination is currently the only effective method to prevent COVID-19, and structural proteins are critical targets for vaccine development. Currently, many vaccines are in clinical trials or are already on the market. This review highlights ongoing advances in the design of prophylactic or therapeutic vaccines against COVID-19, including viral vector vaccines, DNA vaccines, RNA vaccines, live-attenuated vaccines, inactivated virus vaccines, recombinant protein vaccines and bionic nanoparticle vaccines. In addition to traditional inactivated virus vaccines, some novel vaccines based on viral vectors, nanoscience and synthetic biology also play important roles in combating COVID-19. However, many challenges persist in ongoing clinical trials.
Keywords: Coronavirus disease 2019 (COVID-19); Nanoscience; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Synthetic biology; Vaccine.
© 2021. The Author(s).
Conflict of interest statement
The authors have applied for a Chinese patent for a bionic nanoparticle vaccine against COVID-19.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

