Porcine reproductive and respiratory syndrome virus nsp4 positively regulates cellular cholesterol to inhibit type I interferon production
- PMID: 34911669
- PMCID: PMC8758914
- DOI: 10.1016/j.redox.2021.102207
Porcine reproductive and respiratory syndrome virus nsp4 positively regulates cellular cholesterol to inhibit type I interferon production
Abstract
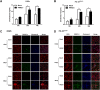
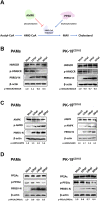
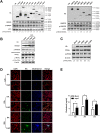
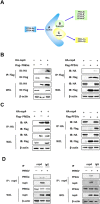
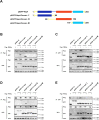
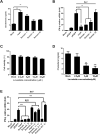
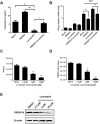
Cellular cholesterol plays an important role in the life cycles of enveloped viruses. Previous studies by our group and other groups have demonstrated that the depletion of cellular cholesterol by methyl-β-cyclodextrin (MβCD) reduces the proliferation of porcine reproductive and respiratory syndrome virus (PRRSV), a porcine Arterivirus that has been devastating the swine industry worldwide for over two decades. However, how PRRSV infection regulates cholesterol synthesis is not fully understood. In this study, we showed that PRRSV infection upregulated the activity of protein phosphatase 2 (PP2A), which subsequently activated 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), the rate-limiting enzyme in the cholesterol synthesis pathway, to increase the levels of cellular cholesterol. By screening the PRRSV-encoded proteins, we showed that nsp4 dominated the upregulation of cellular cholesterol, independently of the 3C-like protease activity of nsp4. A mutation analysis showed that domain I (amino acids 1-80) of PRRSV nsp4 interacted with PR65 alpha (PR65α), the structural subunit, and PP2Ac, the catalytic subunit, of PP2A. Importantly, domain I of nsp4 inhibited Sendai virus-induced interferon β production, and this inhibitory effect was eliminated by Lovastatin, an HMGCR inhibitor, indicating that the upregulation of cellular cholesterol by nsp4 is a strategy used by PRRSV to suppress the antiviral innate immunity of its host. Collectively, we here demonstrated the mechanism by which PRRSV regulates cellular cholesterol synthesis and reported a novel strategy by which PRRSV evades its host's antiviral innate immune response.
Keywords: AMPK; Cholesterol; HMGCR; Interferon; PP2A; PRRSV.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
All the authors declare that there are no conflicts of interest.
Figures
References
-
- Wensvoort G., Terpstra C., Pol J.M., et al. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13(3):121–130. - PubMed
-
- Lunney J.K., Fang Y., Ladinig A., et al. Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2016;4:129–154. - PubMed
-
- Snijder E.J., Kikkert M., Fang Y. Arterivirus molecular biology and pathogenesis. J. Gen. Virol. 2013;94(Pt 10):2141–2163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

