Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study
- PMID: 34911691
- PMCID: PMC8671836
- DOI: 10.1136/bmj-2021-068848
Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study
Abstract
Objectives: To evaluate the effectiveness of the mRNA-1273 vaccine against SARS-CoV-2 variants and assess its effectiveness against the delta variant by time since vaccination.
Design: Test negative case-control study.
Setting: Kaiser Permanente Southern California (KPSC), an integrated healthcare system.
Participants: Adult KPSC members with a SARS-CoV-2 positive test sent for whole genome sequencing or a negative test from 1 March 2021 to 27 July 2021.
Interventions: Two dose or one dose vaccination with mRNA-1273 (Moderna covid-19 vaccine) ≥14 days before specimen collection versus no covid-19 vaccination.
Main outcome measures: Outcomes included infection with SARS-CoV-2 and hospital admission with covid-19. In pre-specified analyses for each variant type, test positive cases were matched 1:5 to test negative controls on age, sex, race/ethnicity, and specimen collection date. Conditional logistic regression was used to compare odds of vaccination among cases versus controls, with adjustment for confounders. Vaccine effectiveness was calculated as (1-odds ratio)×100%.
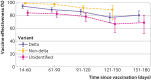
Results: The study included 8153 cases and their matched controls. Two dose vaccine effectiveness was 86.7% (95% confidence interval 84.3% to 88.7%) against infection with the delta variant, 98.4% (96.9% to 99.1%) against alpha, 90.4% (73.9% to 96.5%) against mu, 96-98% against other identified variants, and 79.9% (76.9% to 82.5%) against unidentified variants (that is, specimens that failed sequencing). Vaccine effectiveness against hospital admission with the delta variant was 97.5% (92.7% to 99.2%). Vaccine effectiveness against infection with the delta variant declined from 94.1% (90.5% to 96.3%) 14-60 days after vaccination to 80.0% (70.2% to 86.6%) 151-180 days after vaccination. Waning was less pronounced for non-delta variants. Vaccine effectiveness against delta infection was lower among people aged ≥65 years (75.2%, 59.6% to 84.8%) than those aged 18-64 years (87.9%, 85.5% to 89.9%). One dose vaccine effectiveness was 77.0% (60.7% to 86.5%) against infection with delta.
Conclusions: Two doses of mRNA-1273 were highly effective against all SARS-CoV-2 variants, especially against hospital admission with covid-19. However, vaccine effectiveness against infection with the delta variant moderately declined with increasing time since vaccination.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Moderna for the submitted work; KJB was employed by KPSC during the conduct of the study and is now an adjunct investigator at KPSC; LSS, LQ, BKA, YL, GSL, YT, AF, MA, JET, HST, JHK, and HFT are employees of KPSC, which has been contracted by Moderna to conduct this study; CAT and YDP are employees of and shareholders in Moderna Inc; KJB received funding from GlaxoSmithKline, Dynavax, Pfizer, Gilead, and Seqirus unrelated to this manuscript; LSS received funding from GlaxoSmithKline, Dynavax, and Seqirus unrelated to this manuscript; LQ received funding from GlaxoSmithKline and Dynavax unrelated to this manuscript; BKA received funding from GlaxoSmithKline, Dynavax, Seqirus, and Pfizer unrelated to this manuscript; YL received funding from GlaxoSmithKline, Seqirus, and Pfizer unrelated to this manuscript; GSL received funding from GlaxoSmithKline unrelated to this manuscript; YT received funding from GlaxoSmithKline unrelated to this manuscript; AF received funding from Pfizer, GlaxoSmithKline, and Gilead unrelated to this manuscript; MA received funding from Pfizer unrelated to this manuscript; JET received funding from Pfizer unrelated to this manuscript; HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript; JHK received funding from GlaxoSmithKline unrelated to this manuscript; HFT received funding from GlaxoSmithKline and Seqirus unrelated to this manuscript and also served on advisory boards for Janssen and Pfizer.
Figures

Comment in
-
Covid-19 vaccines, immunity, and boosters.BMJ. 2021 Dec 20;375:n3105. doi: 10.1136/bmj.n3105. BMJ. 2021. PMID: 34930779 No abstract available.
References
-
- United States Food and Drug Administration. Moderna COVID-19 Vaccine. 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- United States Food and Drug Administration. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- World Health Organization. COVID-19 Vaccine Tracker. 2021. https://covid19.trackvaccines.org/agency/who/.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
