MEKK3-TGFβ crosstalk regulates inward arterial remodeling
- PMID: 34911761
- PMCID: PMC8713777
- DOI: 10.1073/pnas.2112625118
MEKK3-TGFβ crosstalk regulates inward arterial remodeling
Abstract
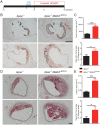
Arterial remodeling is an important adaptive mechanism that maintains normal fluid shear stress in a variety of physiologic and pathologic conditions. Inward remodeling, a process that leads to reduction in arterial diameter, plays a critical role in progression of such common diseases as hypertension and atherosclerosis. Yet, despite its pathogenic importance, molecular mechanisms controlling inward remodeling remain undefined. Mitogen-activated protein kinases (MAPKs) perform a number of functions ranging from control of proliferation to migration and cell-fate transitions. While the MAPK ERK1/2 signaling pathway has been extensively examined in the endothelium, less is known about the role of the MEKK3/ERK5 pathway in vascular remodeling. To better define the role played by this signaling cascade, we studied the effect of endothelial-specific deletion of its key upstream MAP3K, MEKK3, in adult mice. The gene's deletion resulted in a gradual inward remodeling of both pulmonary and systematic arteries, leading to spontaneous hypertension in both vascular circuits and accelerated progression of atherosclerosis in hyperlipidemic mice. Molecular analysis revealed activation of TGFβ-signaling both in vitro and in vivo. Endothelial-specific TGFβR1 knockout prevented inward arterial remodeling in MEKK3 endothelial knockout mice. These data point to the unexpected participation of endothelial MEKK3 in regulation of TGFβR1-Smad2/3 signaling and inward arterial remodeling in artery diseases.
Keywords: MEKK3; atherosclerosis; inward arterial remodeling; pulmonary arterial hypertension; systemic hypertension.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Ward M. R., et al. , Low blood flow after angioplasty augments mechanisms of restenosis: Inward vessel remodeling, cell migration, and activity of genes regulating migration. Arterioscler. Thromb. Vasc. Biol. 21, 208–213 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

