How Compensatory Mechanisms and Adaptive Rewiring Have Shaped Our Understanding of Therapeutic Resistance in Cancer
- PMID: 34911779
- PMCID: PMC9033251
- DOI: 10.1158/0008-5472.CAN-21-3605
How Compensatory Mechanisms and Adaptive Rewiring Have Shaped Our Understanding of Therapeutic Resistance in Cancer
Abstract
Therapeutic resistance to targeted therapies by tumor cells is a common and serious problem in the clinic. Increased understanding of the molecular mechanisms that underly resistance is necessary for the rational design and improvement of effective pharmacologic treatment strategies. The landmark study by O'Reilly and colleagues published in Cancer Research in 2006 provided valuable insights into nongenomic adaptive rewiring and compensatory mechanisms responsible for mediating resistance to targeted inhibition of the PI3K-AKT-mTOR pathway, and how tumor cells regulate signaling pathways via negative feedback loops. These findings have proven fundamental for guiding current efforts to develop effective combination treatments and provided a blueprint for research studies aimed at understanding the intricacies of cellular signaling.See related article by O'Reilly and colleagues, Cancer Res 2006;66:1500-8.
©2021 American Association for Cancer Research.
Figures
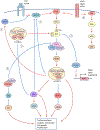
Comment on
-
mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt.Cancer Res. 2006 Feb 1;66(3):1500-8. doi: 10.1158/0008-5472.CAN-05-2925. Cancer Res. 2006. PMID: 16452206 Free PMC article.
References
-
- Shah OJ, Wang Z, Hunter T. Inappropriate Activation of the TSC/Rheb/mTOR/S6K Cassette Induces IRS1/2 Depletion, Insulin Resistance, and Cell Survival Deficiencies. Curr Biol. 2004;14:1650–6. - PubMed
-
- Sun S-Y, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E Survival Pathways by Rapamycin-Mediated Mammalian Target of Rapamycin Inhibition. Cancer Res. 2005;65:7052–8. - PubMed
-
- Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

