Atrial fibrillation and clinical outcomes 1 to 3 years after myocardial infarction
- PMID: 34911791
- PMCID: PMC8679122
- DOI: 10.1136/openhrt-2021-001726
Atrial fibrillation and clinical outcomes 1 to 3 years after myocardial infarction
Abstract
Objective: Atrial fibrillation (AF) and myocardial infarction (MI) are commonly comorbid and associated with adverse outcomes. Little is known about the impact of AF on quality of life and outcomes post-MI. We compared characteristics, quality of life and clinical outcomes in stable patients post-MI with/without AF.
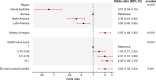
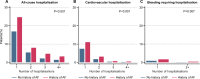
Methods/results: The prospective, international, observational TIGRIS (long Term rIsk, clinical manaGement and healthcare Resource utilization of stable coronary artery dISease) registry included 8406 patients aged ≥50 years with ≥1 atherothrombotic risk factor who were 1-3 years post-MI. Patient characteristics were summarised by history of AF. Quality of life was assessed at baseline using EQ-5D. Clinical outcomes over 2 years of follow-up were compared. History of AF was present in 702/8277 (8.5%) registry patients and incident AF was diagnosed in 244/7575 (3.2%) over 2 years. Those with AF were older and had more comorbidities than those without AF. After multivariable adjustment, patients with AF had lower self-reported quality-of-life scores (EQ-5D UK-weighted index, visual analogue scale, usual activities and pain/discomfort) than those without AF. CHA2DS2-VASc score ≥2 was present in 686/702 (97.7%) patients with AF, although only 348/702 (49.6%) were on oral anticoagulants at enrolment. Patients with AF had higher rates of all-cause hospitalisation (adjusted rate ratio 1.25 [1.06-1.46], p=0.008) over 2 years than those without AF, but similar rates of mortality.
Conclusions: In stable patients post-MI, those with AF were commonly undertreated with oral anticoagulants, had poorer quality of life and had increased risk of clinical outcomes than those without AF.
Trial registration number: ClinicalTrials: NCT01866904.
Keywords: atrial fibrillation; health services; myocardial infarction; stroke.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: APC reports grant funding from the National Institutes of Health. RO has received research grant support from AstraZeneca. SJP has received research grant support from AstraZeneca. DBB has received speaker/consulting honoraria and/or research grant support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, Merck and Sanofi. SY has received speaker/consulting honoraria and/or research grant support from Takeda, Daiichi Sankyo, AstraZeneca, Boehringer Ingelheim and BMS. JCN has received speaker/consulting honoraria and/or research grant support from Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, GSK, Merck, Novartis, Pfizer and Sanofi. SGG has received research grant support (eg, steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (eg, advisory boards) from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier and Valeo Pharma, and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Center for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Center and PERFUSE Research Institute. MGC has received speaker/consulting honoraria and/or research grant support from AstraZeneca, Medtronic, Abiomed and Merit Medical. TS has received speaker/consulting honoraria and/or research grant support from Astellas, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Pfizer and Sanofi. DW has received speaker/consulting honoraria and/or research grant support from AstraZeneca, Bayer, Berlin-Chemie, Biotronik and Novartis. KH and KAS are employees of AstraZeneca. CBG has received consulting honoraria and/or research grant support from Armetheon, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Eli Lilly, Gilead, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Medtronic, Pfizer, Salix Pharmaceuticals, Sanofi, Takeda and The Medicines Company.
Figures



References
-
- Zeymer U, Annemans L, Danchin N, et al. . Impact of known or new-onset atrial fibrillation on 2-year cardiovascular event rate in patients with acute coronary syndromes: results from the prospective EPICOR Registry. Eur Heart J Acute Cardiovasc Care 2019;8:121–9. 10.1177/2048872618769057 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
