Cellular-Resolution Imaging of Bystander Payload Tissue Penetration from Antibody-Drug Conjugates
- PMID: 34911819
- PMCID: PMC8832507
- DOI: 10.1158/1535-7163.MCT-21-0580
Cellular-Resolution Imaging of Bystander Payload Tissue Penetration from Antibody-Drug Conjugates
Abstract
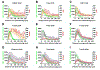
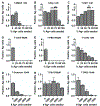
After several notable clinical failures in early generations, antibody-drug conjugates (ADC) have made significant gains with seven new FDA approvals within the last 3 years. These successes have been driven by a shift towards mechanistically informed ADC design, where the payload, linker, drug-to-antibody ratio, and conjugation are increasingly tailored to a specific target and clinical indication. However, fundamental aspects needed for design, such as payload distribution, remain incompletely understood. Payloads are often classified as "bystander" or "nonbystander" depending on their ability to diffuse out of targeted cells into adjacent cells that may be antigen-negative or more distant from tumor vessels, helping to overcome heterogeneous distribution. Seven of the 11 FDA-approved ADCs employ these bystander payloads, but the depth of penetration and cytotoxic effects as a function of physicochemical properties and mechanism of action have not been fully characterized. Here, we utilized tumor spheroids and pharmacodynamic marker staining to quantify tissue penetration of the three major classes of agents: microtubule inhibitors, DNA-damaging agents, and topoisomerase inhibitors. PAMPA data and coculture assays were performed to compare with the 3D tissue culture data. The results demonstrate a spectrum in bystander potential and tissue penetration depending on the physicochemical properties and potency of the payload. Generally, directly targeted cells show a greater response even with bystander payloads, consistent with the benefit of deeper ADC tissue penetration. These results are compared with computational simulations to help scale the data from in vitro and preclinical animal models to the clinic.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures


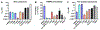

Comment on
-
Selected Articles from This Issue.Mol Cancer Ther. 2022 Feb;21(2):243. doi: 10.1158/1535-7163.MCT-21-2-HI. Mol Cancer Ther. 2022. PMID: 35135870 No abstract available.
References
-
- Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, et al. Antibody-Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clinical cancer research : an official journal of the American Association for Cancer Research 2019;25(18):5441–8 doi 10.1158/1078-0432.CCR-19-0272. - DOI - PubMed
-
- Tumey LN. Next Generation Payloads for ADCs. In: Damelin M, editor. Innovations for Next-Generation Antibody-Drug Conjugates Cham: Springer International Publishing; 2018. p 187–214.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

