Corona Virus Disease 2019 (COVID-19) as a System-Level Infectious Disease With Distinct Sex Disparities
- PMID: 34912345
- PMCID: PMC8667725
- DOI: 10.3389/fimmu.2021.778913
Corona Virus Disease 2019 (COVID-19) as a System-Level Infectious Disease With Distinct Sex Disparities
Abstract
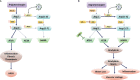
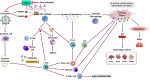
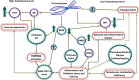
The current global pandemic of the Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) causing COVID-19, has infected millions of people and continues to pose a threat to many more. Angiotensin-Converting Enzyme 2 (ACE2) is an important player of the Renin-Angiotensin System (RAS) expressed on the surface of the lung, heart, kidney, neurons, and endothelial cells, which mediates SARS-CoV-2 entry into the host cells. The cytokine storms of COVID-19 arise from the large recruitment of immune cells because of the dis-synchronized hyperactive immune system, lead to many abnormalities including hyper-inflammation, endotheliopathy, and hypercoagulability that produce multi-organ dysfunction and increased the risk of arterial and venous thrombosis resulting in more severe illness and mortality. We discuss the aberrated interconnectedness and forthcoming crosstalks between immunity, the endothelium, and coagulation, as well as how sex disparities affect the severity and outcome of COVID-19 and harm men especially. Further, our conceptual framework may help to explain why persistent symptoms, such as reduced physical fitness and fatigue during long COVID, may be rooted in the clotting system.
Keywords: COVID-19; coagulation; immunity; interconnectedness; thromboembolism.
Copyright © 2021 Emadi-Baygi, Ehsanifard, Afrashtehpour, Norouzi and Joz-Abbasalian.
Conflict of interest statement
MN was employed by Erythrogen Medical Genetics Lab. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Endothelial cells and SARS-CoV-2: An intimate relationship.Vascul Pharmacol. 2021 Apr;137:106829. doi: 10.1016/j.vph.2021.106829. Epub 2021 Jan 8. Vascul Pharmacol. 2021. PMID: 33422689 Free PMC article. Review.
-
Do inflammaging and coagul-aging play a role as conditions contributing to the co-occurrence of the severe hyper-inflammatory state and deadly coagulopathy during COVID-19 in older people?Exp Gerontol. 2021 Aug;151:111423. doi: 10.1016/j.exger.2021.111423. Epub 2021 May 26. Exp Gerontol. 2021. PMID: 34048906 Free PMC article. Review.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update.High Blood Press Cardiovasc Prev. 2021 Mar;28(2):129-139. doi: 10.1007/s40292-021-00439-9. Epub 2021 Feb 26. High Blood Press Cardiovasc Prev. 2021. PMID: 33635533 Free PMC article. Review.
-
Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19).J Pathol. 2020 Jul;251(3):228-248. doi: 10.1002/path.5471. Epub 2020 Jun 10. J Pathol. 2020. PMID: 32418199 Free PMC article. Review.
Cited by
-
Heparanase Is a Putative Mediator of Endothelial Glycocalyx Damage in COVID-19 - A Proof-of-Concept Study.Front Immunol. 2022 Jun 10;13:916512. doi: 10.3389/fimmu.2022.916512. eCollection 2022. Front Immunol. 2022. PMID: 35757776 Free PMC article.
-
Characterization of Clinical Features of Hospitalized Patients Due to the SARS-CoV-2 Infection in the Absence of Comorbidities Regarding the Sex: An Epidemiological Study of the First Year of the Pandemic in Brazil.Int J Environ Res Public Health. 2022 Jul 22;19(15):8895. doi: 10.3390/ijerph19158895. Int J Environ Res Public Health. 2022. PMID: 35897265 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

