Bevacizumab Increases Endothelin-1 Production via Forkhead Box Protein O1 in Human Glomerular Microvascular Endothelial Cells In Vitro
- PMID: 34912580
- PMCID: PMC8668358
- DOI: 10.1155/2021/8381115
Bevacizumab Increases Endothelin-1 Production via Forkhead Box Protein O1 in Human Glomerular Microvascular Endothelial Cells In Vitro
Abstract
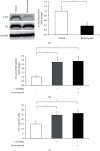
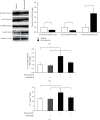
Molecular mechanisms underlying the nephrotoxicity associated with bevacizumab are unclear. Endothelin-1 (ET-1) is involved in podocyte injury and proteinuria, and its level increases in most cases of kidney disorders. Forkhead box protein O1 (FoxO1), a transcription factor, is a major determinant of ET-1 promoter activation and is regulated by protein kinase B (Akt) phosphorylation-dependent nuclear exclusion. We evaluated the effect of bevacizumab on ET-1 production in human glomerular microvascular endothelial cells (hGECs). We analyzed the changes in the mRNA and protein levels of ET-1 in hGECs treated with bevacizumab using real-time reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay. Changes in the protein levels and phosphorylation status of Akt and FoxO1 in hGECs treated with bevacizumab were analyzed by western blotting. After cell lysis, FoxO1 protein was isolated from the cytoplasmic and nuclear fractions. We also investigated the effects of AS1842856 (a FoxO1 inhibitor) on bevacizumab-induced ET-1 production. Bevacizumab significantly and dose-dependently increased the mRNA and protein levels of ET-1 in hGECs (p < 0.05). Bevacizumab treatment also led to a decrease in phosphorylated Akt protein levels. Inhibition of Akt activity by LY294002 promoted ET-1 production. Bevacizumab also induced an increase in FoxO1 protein levels in the nucleus. Inhibition of FoxO1 activity by AS1842856 resulted in decreased ET-1 levels in bevacizumab-treated hGECs. ET-1 axis activation, Akt inactivation, and FoxO1 nuclear localization are the molecular mechanisms underlying bevacizumab-induced nephrotoxicity. Therefore, inhibition of renal ET-1 production could be a promising approach to protect against or treat bevacizumab-induced nephrotoxicity.
Copyright © 2021 Satoru Nihei et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

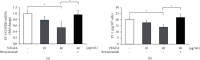

References
-
- Rini B. I., Halabi S., Rosenberg J. E., et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of Clinical Oncology . 2010;28(13):2137–2143. doi: 10.1200/jco.2009.26.5561. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

