Sensitive LC-MS/MS Methods for Amphotericin B Analysis in Cerebrospinal Fluid, Plasma, Plasma Ultrafiltrate, and Urine: Application to Clinical Pharmacokinetics
- PMID: 34912784
- PMCID: PMC8666623
- DOI: 10.3389/fchem.2021.782131
Sensitive LC-MS/MS Methods for Amphotericin B Analysis in Cerebrospinal Fluid, Plasma, Plasma Ultrafiltrate, and Urine: Application to Clinical Pharmacokinetics
Abstract
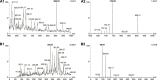
Neurocryptococcosis, a meningoencephalitis caused by Cryptococcus spp, is treated with amphotericin B (AmB) combined with fluconazole. The integrity of the brain-blood barrier and the composition of the cerebrospinal fluid (CSF) may change due to infectious and/or inflammatory diseases such as neurocryptococcosis allowing for the penetration of AmB into the central nervous system. The present study aimed to develop LC-MS/MS methods capable of quantifying AmB in CSF at any given time of the treatment in addition to plasma, plasma ultrafiltrate, with sensitivity compatible with the low concentrations of AmB reported in the CSF. The methods were successfully validated in the four matrices (25 μl, 5-1,000 ng ml-1 for plasma or urine; 100 μl, 0.625-250 ng ml-1 for plasma ultrafiltrate; 100 μl, 0.1-250 ng ml-1 for CSF) using protein precipitation. The methods were applied to investigate the pharmacokinetics of AmB following infusions of 100 mg every 24 h for 16 days administered as a lipid complex throughout the treatment of a neurocryptococcosis male patient. The methods allowed for a detailed description of the pharmacokinetic parameters in the assessed patient in the beginning (4th day) and end of the treatment with AmB (16th day), with total clearances of 7.21 and 4.25 L h-1, hepatic clearances of 7.15 and 4.22 L h-1, volumes of distribution of 302.94 and 206.89 L, and unbound fractions in plasma ranging from 2.26 to 3.25%. AmB was quantified in two CSF samples collected throughout the treatment with concentrations of 12.26 and 18.45 ng ml-1 on the 8th and 15th days of the treatment, respectively. The total concentration of AmB in plasma was 31 and 20 times higher than in CSF. The unbound concentration in plasma accounted for 77 and 44% of the respective concentrations in CSF. In conclusion, the present study described the most complete and sensitive method for AmB analysis in plasma, plasma ultrafiltrate, urine, and CSF applied to a clinical pharmacokinetic study following the administration of the drug as a lipid complex in one patient with neurocryptococcosis. The method can be applied to investigate the pharmacokinetics of AmB in CSF at any given time of the treatment.
Keywords: LC-MS/MS; amphotericin B; cerebrospinal fluid; neurocryptococcosis; pharmacokinetics; plasma; unbound fraction; urine.
Copyright © 2021 Pippa, Marques, Silva, Vilar, de Haes, Fonseca, Martinez, Coelho, Wichert-Ana and Lanchote.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Adedoyin A., Swenson C. E., Bolcsak L. E., Hellmann A., Radowska D., Horwith G., et al. (2000). A Pharmacokinetic Study of Amphotericin B Lipid Complex Injection (Abelcet) in Patients with Definite or Probable Systemic Fungal Infections. Antimicrob. Agents Chemother. 44, 2900–2902. 10.1128/aac.44.10.2900-2902.2000 - DOI - PMC - PubMed
-
- Ayestarán A., López R. M., Montoro J. B., Estíbalez A., Pou L., Julià A., et al. (1996). Pharmacokinetics of Conventional Formulation versus Fat Emulsion Formulation of Amphotericin B in a Group of Patients with Neutropenia. Antimicrob. Agents Chemother. 40, 609–612. 10.1128/AAC.40.3.609 - DOI - PMC - PubMed
-
- Beggs, W. H. (1978). Kinetics of Amphotericin B Decay in a Liquid Medium and Characterization of the Decay Process. Curr. Microbiol. 1, 301–304. 10.1007/BF02601687 - DOI
-
- Bekersky I., Fielding R. M., Dressler D. E., Lee J. W., Buell D. N., Walsh T. J. (2002a). Pharmacokinetics, Excretion, and Mass Balance of Liposomal Amphotericin B (AmBisome) and Amphotericin B Deoxycholate in Humans. Antimicrob. Agents Chemother. 46, 828–833. 10.1128/AAC.46.3.828-833.2002 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

