Baseline type 2 biomarker levels and response to tezepelumab in severe asthma
- PMID: 34913186
- PMCID: PMC9306691
- DOI: 10.1111/all.15197
Baseline type 2 biomarker levels and response to tezepelumab in severe asthma
Abstract
Background: Tezepelumab is a human monoclonal antibody that blocks activity of thymic stromal lymphopoietin (TSLP). In the phase IIb PATHWAY study (NCT02054130), tezepelumab significantly reduced annualized asthma exacerbation rates (AAERs) versus placebo in adults with severe, uncontrolled asthma. We evaluated the effects of tezepelumab in reducing type 2 (T2) inflammatory biomarker levels in the PATHWAY population, and the relationship between baseline T2 biomarker levels and AAER.
Methods: Adults with severe, uncontrolled asthma (n = 550) were randomized to tezepelumab (70 mg or 210 mg every 4 weeks, or 280 mg every 2 weeks) or placebo for 52 weeks. Blood eosinophil count, fractional exhaled nitric oxide (FeNO), and serum total immunoglobulin (Ig)E, interleukin (IL)-5, IL-13, periostin, thymus and activation-regulated chemokine (TARC), and TSLP were measured at baseline and over 52 weeks. AAERs were analyzed by baseline threshold (high/low) biomarker levels.
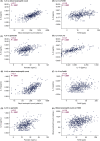
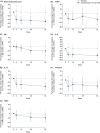
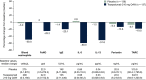
Results: Positive correlations were observed between T2 inflammatory biomarkers (blood eosinophil count, FeNO, IL-5, IL-13 and periostin) at baseline. At Week 52, treatment with tezepelumab 210 mg reduced all biomarker levels measured from baseline versus placebo. Exacerbations were reduced by 55-83% in the pooled tezepelumab cohort versus placebo, irrespective of baseline blood eosinophil count, FeNO, or serum total IgE, IL-5, IL-13, periostin, TARC, or TSLP, when these biomarkers were assessed individually.
Conclusion: At baseline, positive correlations between specific T2 inflammatory biomarkers were observed. Tezepelumab reduced multiple T2 inflammatory biomarkers, which indicates decreased airway inflammation, and reduced exacerbations irrespective of baseline T2 biomarker profiles in patients with severe asthma.
Keywords: asthma; biomarkers; inflammation.
© 2022 AstraZeneca and Amgen Inc. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Corren received grants from AstraZeneca during the conduct of the study. He has also received grants from Genentech, Novartis, Optinose, Regeneron, Sanofi, and Teva, and has received personal fees from AstraZeneca, Genentech, Regeneron, and Sanofi. Ms Pham, Dr. Ren, Dr. Colice, and Dr. Griffiths are employees of AstraZeneca and own stock and stock options in AstraZeneca. Dr. Garcia Gil was an employee of AstraZeneca at the time of this study; she is a present employee of Almirall. Ms Sałapa is an employee of AstraZeneca. Dr. Parnes is an employee of Amgen Inc., and owns stock and stock options in Amgen Inc.
Figures

References
-
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. - PubMed
-
- Global Initiative for Asthma . Global initiative for asthma management and prevention 2020 guidelines. https://ginasthma.org/wp‐content/uploads/2020/06/GINA‐2020‐report_20_06_.... Assessed February 26, 2021.
-
- Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896‐902. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

