Single-Ion Lithium Conducting Polymers with High Ionic Conductivity Based on Borate Pendant Groups
- PMID: 34913231
- PMCID: PMC9306921
- DOI: 10.1002/anie.202114024
Single-Ion Lithium Conducting Polymers with High Ionic Conductivity Based on Borate Pendant Groups
Abstract
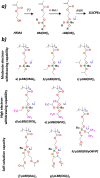
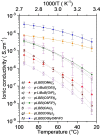
A family of single-ion lithium conducting polymer electrolytes based on highly delocalized borate groups is reported. The effect of the nature of the substituents on the boron atom on the ionic conductivity of the resultant methacrylic polymers was analyzed. To the best of our knowledge the lithium borate polymers endowed with flexible and electron-withdrawing substituents presents the highest ionic conductivity reported for a lithium single-ion conducting homopolymer (1.65×10-4 S cm-1 at 60 °C). This together with its high lithium transference number t =0.93 and electrochemical stability window of 4.2 V vs Li0 /Li+ show promise for application in lithium batteries. To illustrate this, a lithium borate monomer was integrated into a single-ion gel polymer electrolyte which showed good performance on lithium symmetrical cells (<0.85 V at ±0.2 mA cm-2 for 175 h).
Keywords: Boron-Based Polymers; Lithium Batteries; Polymer Electrolytes; Polymerizable Boron-Lithium Salts.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Oh K.-S., Kim J.-H., Kim S.-H., Oh D., Han S.-P., Jung K., Wang Z., Shi L., Su Y., Yim T., Yuan S., Lee S.-Y., Adv. Energy Mater. 2021, 11, 2101813.
-
- Zhou D., Tkacheva A., Tang X., Sun B., Shanmukaraj D., Li P., Zhang F., Armand M., Wang G., Angew. Chem. Int. Ed. 2019, 58, 6001–6006; - PubMed
- Angew. Chem. 2019, 131, 6062–6067.
-
- Khan I. M., Fish D., Delaviz Y., Smid J., Makromol. Chem. 1989, 190, 1069–1078.
-
- Strauss E., Menkin S., Golodnitsky D., J. Solid State Electrochem. 2017, 21, 1879–1905.
-
- Zhou D., Shanmukaraj D., Tkacheva A., Armand M., Wang G., Chem 2019, 5, 2326–2352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

