Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial
- PMID: 34914568
- PMCID: PMC8689623
- DOI: 10.1177/03000605211062770
Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial
Abstract
Objective: Heart failure (HF) is a common and highly morbid cardiovascular disorder. Oxidative stress worsens HF, and uric acid (UA) is a useful oxidative stress marker. The novel anti-hyperuricemic drug febuxostat is a potent non-purine selective xanthine oxidase inhibitor. The present study examined the UA-lowering and prognostic effects of febuxostat in patients with HF compared with conventional allopurinol.
Methods: This multicenter, randomized trial included 263 patients with chronic HF who were randomly assigned to two groups and received allopurinol or febuxostat (UA >7.0 mg/dL). All patients were followed up for 3 years after enrollment.
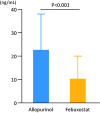
Results: There were no significant differences in baseline clinical characteristics between the two groups. The UA level was significantly decreased after 3 years of drug administration compared with the baseline in both groups. Urine levels of the oxidative stress marker 8-hydroxy-2'-deoxyguanosine were lower in the febuxostat group than in the allopurinol group (11.0 ± 9.6 vs. 22.9 ± 15.9 ng/mL), and the rate of patients free from hospitalization due to worsening HF tended to be higher in the febuxostat group than in the allopurinol group (89.0% vs. 83.0%).
Conclusions: Febuxostat is potentially more effective than allopurinol for treating patients with chronic HF and hyperuricemia.This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (https://www.umin.ac.jp/ctr/; ID: 000009817).
Keywords: Heart failure; febuxostat; oxidative stress; prognosis; uric acid; worsening heart failure.
Conflict of interest statement
Figures





References
-
- Yancy CW, Jessup M, Bozkurt B, et al.. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, et al.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. - PubMed
-
- Ayoub KF, Pothineni NVK, Rutland J, et al.. Immunity, Inflammation, and Oxidative Stress in Heart Failure: Emerging Molecular Targets. Cardiovasc Drugs Ther 2017; 31: 593–608. - PubMed
-
- Takeishi Y. Biomarkers in heart failure. Int Heart J 2014; 55: 474–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

