Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia
- PMID: 34914695
- PMCID: PMC8675653
- DOI: 10.1371/journal.pbio.3001480
Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia
Erratum in
-
Correction: Mutations in LRRK2 linked to Parkinson disease sequester Rab8a to damaged lysosomes and regulate transferrin-mediated iron uptake in microglia.PLoS Biol. 2022 May 4;20(5):e3001621. doi: 10.1371/journal.pbio.3001621. eCollection 2022 May. PLoS Biol. 2022. PMID: 35507910 Free PMC article.
Abstract
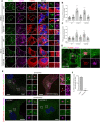
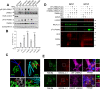
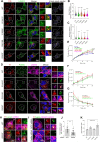
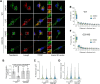
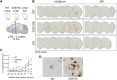
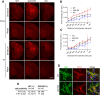
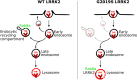
Mutations in leucine-rich repeat kinase 2 (LRRK2) cause autosomal dominant Parkinson disease (PD), while polymorphic LRRK2 variants are associated with sporadic PD. PD-linked mutations increase LRRK2 kinase activity and induce neurotoxicity in vitro and in vivo. The small GTPase Rab8a is a LRRK2 kinase substrate and is involved in receptor-mediated recycling and endocytic trafficking of transferrin, but the effect of PD-linked LRRK2 mutations on the function of Rab8a is poorly understood. Here, we show that gain-of-function mutations in LRRK2 induce sequestration of endogenous Rab8a to lysosomes in overexpression cell models, while pharmacological inhibition of LRRK2 kinase activity reverses this phenotype. Furthermore, we show that LRRK2 mutations drive association of endocytosed transferrin with Rab8a-positive lysosomes. LRRK2 has been nominated as an integral part of cellular responses downstream of proinflammatory signals and is activated in microglia in postmortem PD tissue. Here, we show that iPSC-derived microglia from patients carrying the most common LRRK2 mutation, G2019S, mistraffic transferrin to lysosomes proximal to the nucleus in proinflammatory conditions. Furthermore, G2019S knock-in mice show a significant increase in iron deposition in microglia following intrastriatal LPS injection compared to wild-type mice, accompanied by striatal accumulation of ferritin. Our data support a role of LRRK2 in modulating iron uptake and storage in response to proinflammatory stimuli in microglia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

