Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients
- PMID: 34914868
- PMCID: PMC8693688
- DOI: 10.1056/NEJMoa2116044
Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients
Abstract
Background: New treatments are needed to reduce the risk of progression of coronavirus disease 2019 (Covid-19). Molnupiravir is an oral, small-molecule antiviral prodrug that is active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
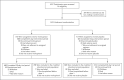
Methods: We conducted a phase 3, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of treatment with molnupiravir started within 5 days after the onset of signs or symptoms in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed Covid-19 and at least one risk factor for severe Covid-19 illness. Participants in the trial were randomly assigned to receive 800 mg of molnupiravir or placebo twice daily for 5 days. The primary efficacy end point was the incidence hospitalization or death at day 29; the incidence of adverse events was the primary safety end point. A planned interim analysis was performed when 50% of 1550 participants (target enrollment) had been followed through day 29.
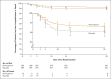
Results: A total of 1433 participants underwent randomization; 716 were assigned to receive molnupiravir and 717 to receive placebo. With the exception of an imbalance in sex, baseline characteristics were similar in the two groups. The superiority of molnupiravir was demonstrated at the interim analysis; the risk of hospitalization for any cause or death through day 29 was lower with molnupiravir (28 of 385 participants [7.3%]) than with placebo (53 of 377 [14.1%]) (difference, -6.8 percentage points; 95% confidence interval [CI], -11.3 to -2.4; P = 0.001). In the analysis of all participants who had undergone randomization, the percentage of participants who were hospitalized or died through day 29 was lower in the molnupiravir group than in the placebo group (6.8% [48 of 709] vs. 9.7% [68 of 699]; difference, -3.0 percentage points; 95% CI, -5.9 to -0.1). Results of subgroup analyses were largely consistent with these overall results; in some subgroups, such as patients with evidence of previous SARS-CoV-2 infection, those with low baseline viral load, and those with diabetes, the point estimate for the difference favored placebo. One death was reported in the molnupiravir group and 9 were reported in the placebo group through day 29. Adverse events were reported in 216 of 710 participants (30.4%) in the molnupiravir group and 231 of 701 (33.0%) in the placebo group.
Conclusions: Early treatment with molnupiravir reduced the risk of hospitalization or death in at-risk, unvaccinated adults with Covid-19. (Funded by Merck Sharp and Dohme; MOVe-OUT ClinicalTrials.gov number, NCT04575597.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
Molnupiravir - A Step toward Orally Bioavailable Therapies for Covid-19.N Engl J Med. 2022 Feb 10;386(6):592-593. doi: 10.1056/NEJMe2117814. Epub 2021 Dec 16. N Engl J Med. 2022. PMID: 34914869 Free PMC article. No abstract available.
-
Will molnupiravir be a game changer in our efforts to safe COVID-19 outpatients?Pol Arch Intern Med. 2022 Jan 28;132(1):16183. doi: 10.20452/pamw.16183. Epub 2022 Jan 3. Pol Arch Intern Med. 2022. PMID: 34978394 No abstract available.
-
Molnupiravir for Covid-19 in Nonhospitalized Patients.N Engl J Med. 2022 Mar 31;386(13):e32. doi: 10.1056/NEJMc2201612. Epub 2022 Mar 16. N Engl J Med. 2022. PMID: 35294804 No abstract available.
-
Molnupiravir for Covid-19 in Nonhospitalized Patients.N Engl J Med. 2022 Mar 31;386(13):e32. doi: 10.1056/NEJMc2201612. Epub 2022 Mar 16. N Engl J Med. 2022. PMID: 35294805 No abstract available.
-
Molnupiravir for Covid-19 in Nonhospitalized Patients.N Engl J Med. 2022 Mar 31;386(13):e32. doi: 10.1056/NEJMc2201612. Epub 2022 Mar 16. N Engl J Med. 2022. PMID: 35294806 No abstract available.
-
Molnupiravir for Covid-19 in Nonhospitalized Patients.N Engl J Med. 2022 Mar 31;386(13):e32. doi: 10.1056/NEJMc2201612. Epub 2022 Mar 16. N Engl J Med. 2022. PMID: 35294807 No abstract available.
-
In nonhospitalized, unvaccinated adults with COVID-19, molnupiravir reduced hospitalization or death at 29 d.Ann Intern Med. 2022 Apr;175(4):JC40. doi: 10.7326/J22-0017. Epub 2022 Apr 5. Ann Intern Med. 2022. PMID: 35377719
References
-
- WHO coronavirus (COVID-19) dashboard. Geneva: World Health Organization, 2021. (https://covid19.who.int).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
