Determining anatomical and electrophysiological detail requirements for computational ventricular models of porcine myocardial infarction
- PMID: 34915331
- PMCID: PMC8819160
- DOI: 10.1016/j.compbiomed.2021.105061
Determining anatomical and electrophysiological detail requirements for computational ventricular models of porcine myocardial infarction
Abstract
Background: Computational models of the heart built from cardiac MRI and electrophysiology (EP) data have shown promise for predicting the risk of and ablation targets for myocardial infarction (MI) related ventricular tachycardia (VT), as well as to predict paced activation sequences in heart failure patients. However, most recent studies have relied on low resolution imaging data and little or no EP personalisation, which may affect the accuracy of model-based predictions.
Objective: To investigate the impact of model anatomy, MI scar morphology, and EP personalisation strategies on paced activation sequences and VT inducibility to determine the level of detail required to make accurate model-based predictions.
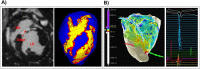
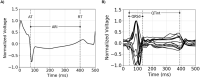
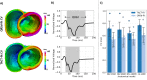
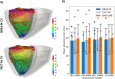
Methods: Imaging and EP data were acquired from a cohort of six pigs with experimentally induced MI. Computational models of ventricular anatomy, incorporating MI scar, were constructed including bi-ventricular or left ventricular (LV) only anatomy, and MI scar morphology with varying detail. Tissue conductivities and action potential duration (APD) were fitted to 12-lead ECG data using the QRS duration and the QT interval, respectively, in addition to corresponding literature parameters. Paced activation sequences and VT induction were simulated. Simulated paced activation and VT inducibility were compared between models and against experimental data.
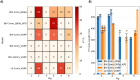
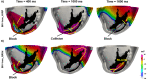
Results: Simulations predict that the level of model anatomical detail has little effect on simulated paced activation, with all model predictions comparing closely with invasive EP measurements. However, detailed scar morphology from high-resolution images, bi-ventricular anatomy, and personalized tissue conductivities are required to predict experimental VT outcome.
Conclusion: This study provides clear guidance for model generation based on clinical data. While a representing high level of anatomical and scar detail will require high-resolution image acquisition, EP personalisation based on 12-lead ECG can be readily incorporated into modelling pipelines, as such data is widely available.
Keywords: Cardiac electrophysiology; Myocardial infarction; Patient-specific models; Ventricular tachycardia.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

