Design, synthesis, and biological evaluation of biotinylated colchicine derivatives as potential antitumor agents
- PMID: 34915785
- PMCID: PMC8725855
- DOI: 10.1080/14756366.2021.2013832
Design, synthesis, and biological evaluation of biotinylated colchicine derivatives as potential antitumor agents
Erratum in
-
Correction.J Enzyme Inhib Med Chem. 2022 Dec;37(1):514. doi: 10.1080/14756366.2022.2024999. J Enzyme Inhib Med Chem. 2022. PMID: 34986713 Free PMC article. No abstract available.
Abstract
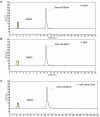
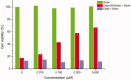
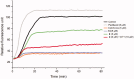
Chemical drug design based on the biochemical characteristics of cancer cells has become an important strategy for discovering new anti-tumour drugs to improve tumour targeting effects and reduce off-target toxicities. Colchicine is one of the most prominent and historically microtubule-targeting drugs, but its clinical applications are hindered by notorious adverse effects. In this study, we presented a novel tumour-specific conjugate 9 that consists of deacetylcolchicine (Deac), biotin, and a cleavable disulphide linker. 9 was found to exhibit potent anti-tumour activity and exerted higher selectivity between tumour and nontarget cells than Deac. The targeting moiety biotin might enhance the transport capability and selectivity of 9 to tumour cells via biotin receptor-mediated endocytosis. The tubulin polymerisation activity of 9 (with DTT) was close to the parent drug Deac. These preliminary results suggested that 9 is a high potency and reduced toxicity antitumor agent and worthy of further investigation.
Keywords: Colchicine; adverse effects; biotin; conjugate; disulphide bond.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


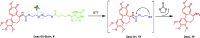


References
-
- Chabner BA, Roberts TG. Jr. , Timeline: chemotherapy and the war on cancer. Nat Rev Cancer 2005;5:65–72. - PubMed
-
- Chan Y, Wong T, Byrne F, et al. . Acid-labile core cross-linked micelles for pH-triggered release of antitumor drugs. Biomacromolecules 2008;9:1826–36. - PubMed
-
- Liu P, Xu J, Yan D, et al. . A DT-diaphorase responsive theranostic prodrug for diagnosis, drug release monitoring and therapy. Chem Commun 2015;51:9567–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
